Pioglitazone hydrochloride p-aminosalicylic acid eutectic crystal, preparation method, composition and application thereof
A technology of pioglitazone hydrochloride and p-aminosalicylic acid, which is applied in the field of medicine, can solve the problems of no pioglitazone hydrochloride eutectic patents or literature reports, low dissolution rate, low dissolution rate, etc., and achieve the effect of significant biological absorption advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Preparation method 1 of pioglitazone hydrochloride and p-aminosalicylic acid cocrystal:
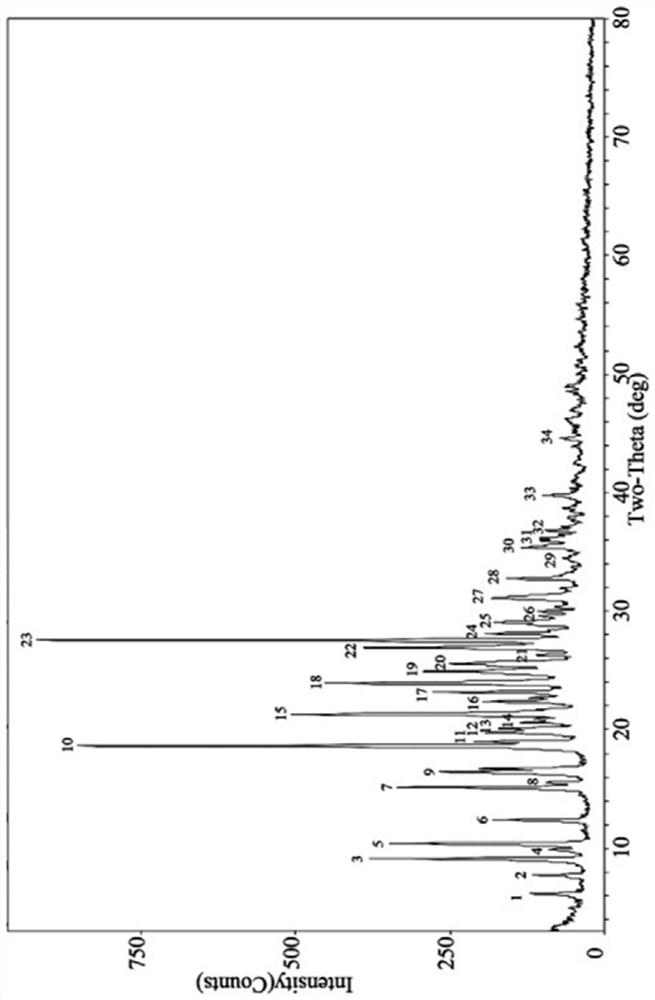
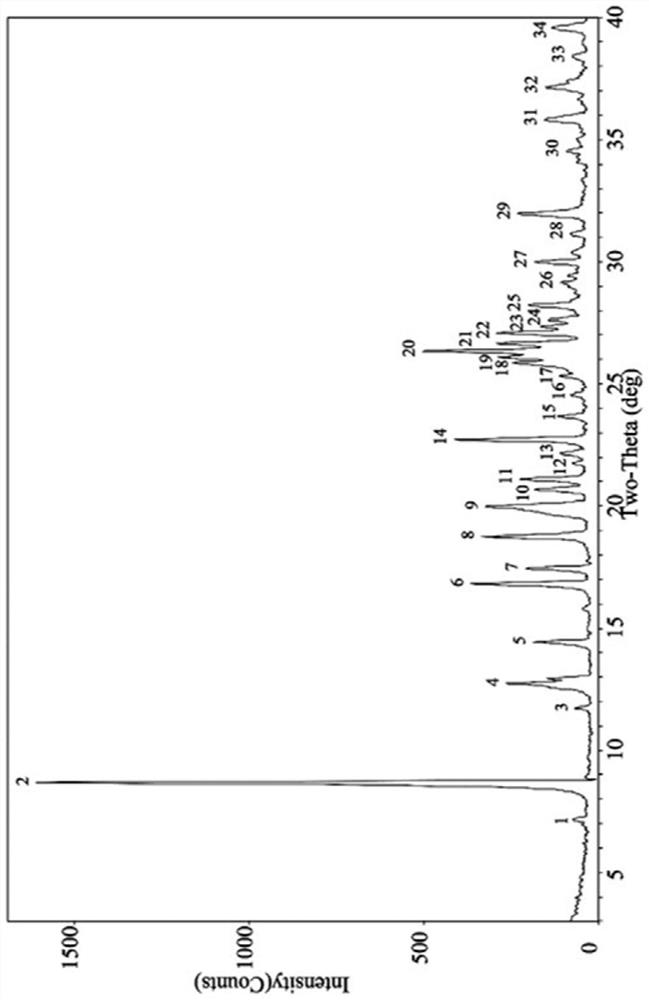
[0061] According to the table below, take appropriate amount of pioglitazone hydrochloride and p-aminosalicylic acid (molar ratio 1:1) into a mortar, add appropriate amount of organic solvent, manually grind for an appropriate time, and dry at a certain temperature. Carry out powder X-ray diffraction analysis to it, its diffraction pattern and figure 1 Consistent, show that the obtained sample is pioglitazone hydrochloride and p-aminosalicylic acid eutectic.
[0062] Table 3 The experimental conditions example of pioglitazone hydrochloride and p-aminosalicylic acid cocrystal preparation method 1
[0063]
[0064] Preparation method 2 of pioglitazone hydrochloride and p-aminosalicylic acid cocrystal:
[0065] As shown in the table below, put appropriate amount of pioglitazone hydrochloride and p-aminosalicylic acid (molar ratio 1:1) into a ball mill jar, add an appropriate amou...
Embodiment 2
[0073] Solubility characteristics of pioglitazone hydrochloride and p-aminosalicylic acid co-crystal:
[0074] Pioglitazone hydrochloride belongs to the poor medicine of water solubility, uses 0.2% SDS aqueous solution to carry out experiment, solvent system has significant difference to the dissolution rate of pioglitazone hydrochloride and p-aminosalicylic acid cocrystal and two kinds of substances of pioglitazone hydrochloride (f 2 =26.23)( Figure 6 ). Determination with reference to the solubility determination method ("Technical Guidelines for Dissolution Test of Ordinary Oral Solid Preparations (First Draft)", 2012, October Center for Drug Evaluation). Adopt high performance liquid chromatography, utilize the chromatographic peak area data of sample to calculate the concentration of sample dissolution, take time as abscissa, and solvent concentration as ordinate to draw solubility curve respectively, and data are as shown in the following table:
[0075] The dissoluti...
Embodiment 3
[0080] The absorption characteristics and plasma concentration characteristics of pioglitazone hydrochloride and p-aminosalicylic acid co-crystal in rats:
[0081] SD rats were randomly divided into groups, 5 in each group, free to drink water, and after fasting for 12 hours, the weight of the rats was weighed, according to 20 mg·kg -1 Pioglitazone hydrochloride dosage calculation, pioglitazone hydrochloride and pioglitazone hydrochloride and p-aminosalicylic acid eutectic samples are packed in the solid dispenser, and the powder is directly inserted into the stomach of rats through the oral cavity. At 15min, 30min, 1h, 1.5h, 2h, 2.5h, 3h, 4h, 6h, 8h, 10h, 12h, 24h, 36h after administration, blood was taken from the inner canthus of the eye and placed in a heparinized tube. Centrifuge at 6500rpm for 10min, and freeze in a -40°C refrigerator until testing. Precisely draw 40 μL of heparin-anticoagulated plasma, put it in a 1.5 mL centrifuge tube, add 10 μL of internal standard ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com