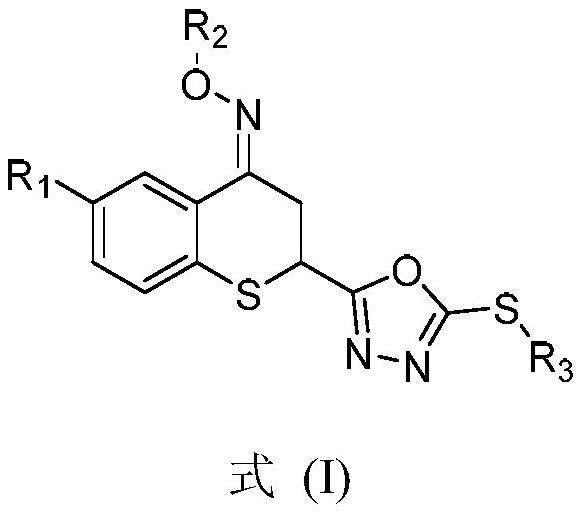
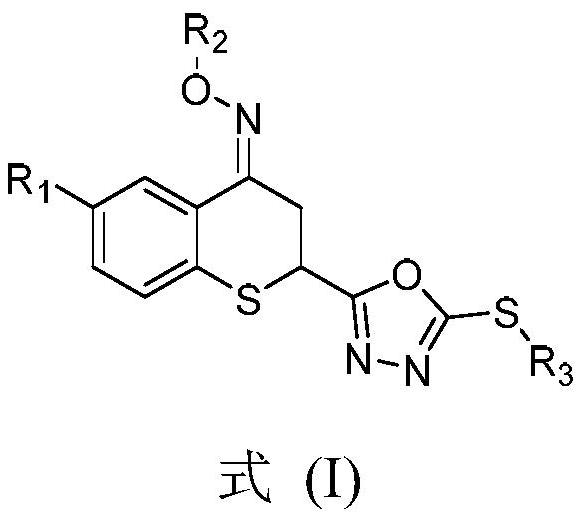
Preparation method and application of thiochroman-4-ketone derivative containing 1, 3, 4-oxadiazole thioether and oxime ether structure
A technology of oxadiazole thioether and thiochroman, applied in the field of preparation and application of thiochroman-4-one derivatives containing 1,3,4-oxadiazole thioether and oxime ether structure, can solve the problem of Wide distribution, microbial and insect poisoning, rapid onset, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
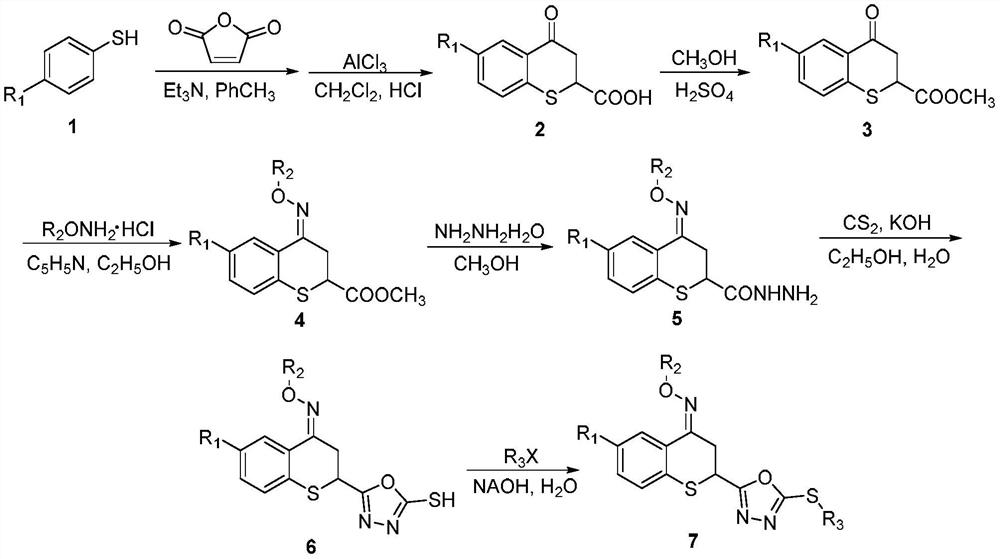
[0052] The present invention uses compound 6-chloro-2-(5-methylthio-1,3,4-oxadiazol-2-yl)-4-methyloxime ether group thiochroman-4-one (compound 7a) The preparation of the target compound is described as an example, and other different substituents are synthesized as a reference.
[0053] The preparation method of 6-chloro-2-(5-methylthio-1,3,4-oxadiazol-2-yl)-4-methyloxime ether group thiochroman-4-one (compound 7a), Include the following steps:
[0054] (1) Synthesis of intermediate 6-chloro-2-carboxythiochroman-4-one
[0055] Add 50mmol of 4-chlorothiophenol, 75mmol of maleic anhydride, and 50mL of toluene to a 500mL flask successively, and dropwise add 5mL of toluene solution containing 2 drops of triethylamine in an oil bath at 50°C. After the addition is completed, The reaction was carried out at 70°C in an oil bath for 4h. After the reaction was over, the toluene was removed under reduced pressure, and the residue was dissolved in 60 mL of dichloromethane. Under ice-...
Embodiment 2
[0067] The preparation method of 6-chloro-2-(5-benzylthio-1,3,4-oxadiazol-2-yl)-4-methyloxime ether group thiochroman-4-one (compound 7b), Include the following steps:
[0068] Referring to the steps of Example 1, the corresponding reactants and reaction parameters were modified to obtain compound 7b.
Embodiment 3
[0070] 6-chloro-2-(5-(4-chlorobenzylthio)-1,3,4-oxadiazol-2-yl)-4-methyloxime ether thiochroman-4-one (compound 7c ) preparation method, comprising the following steps:
[0071] Refer to the steps of Example 1 to modify the corresponding reactants and reaction parameters to obtain compound 7c.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ec50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com