Organic photoelectric material based on perylene bisimide derivative and preparation method thereof
A technology of organic photoelectric materials and perylene imides, which is applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve problems such as the lack of electroluminescent materials, achieve less harsh reaction conditions, lower requirements for instruments and equipment, and synthesize The effect of a short route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] step 1:
[0024]
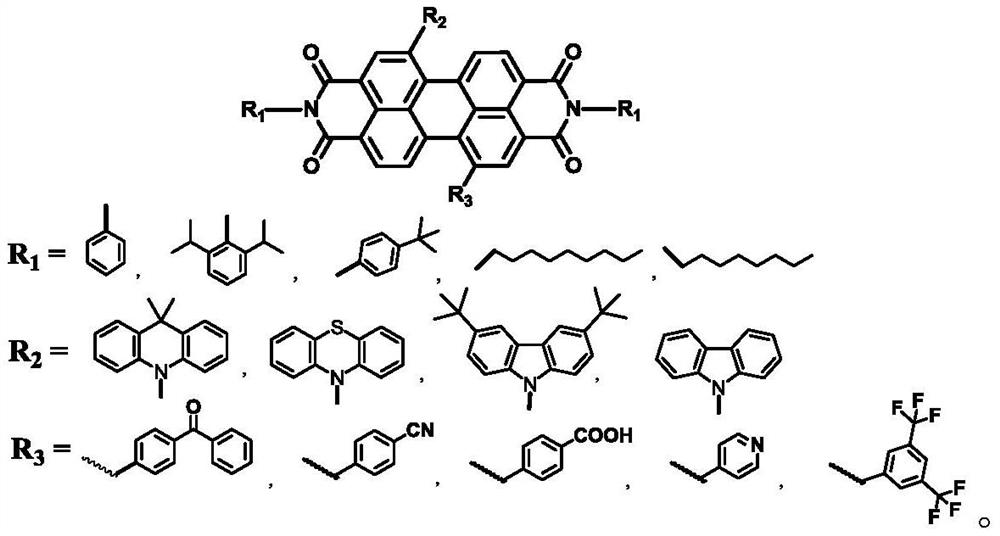
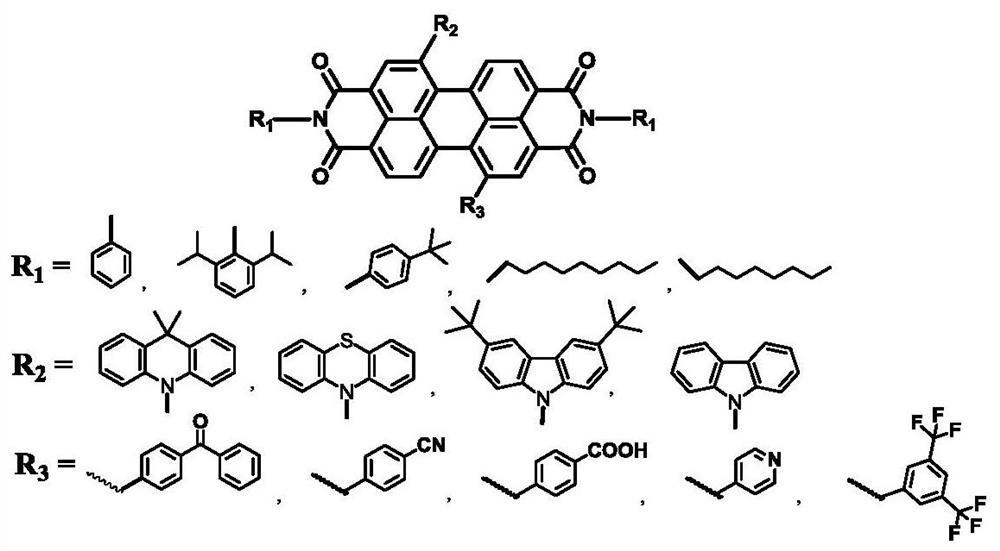
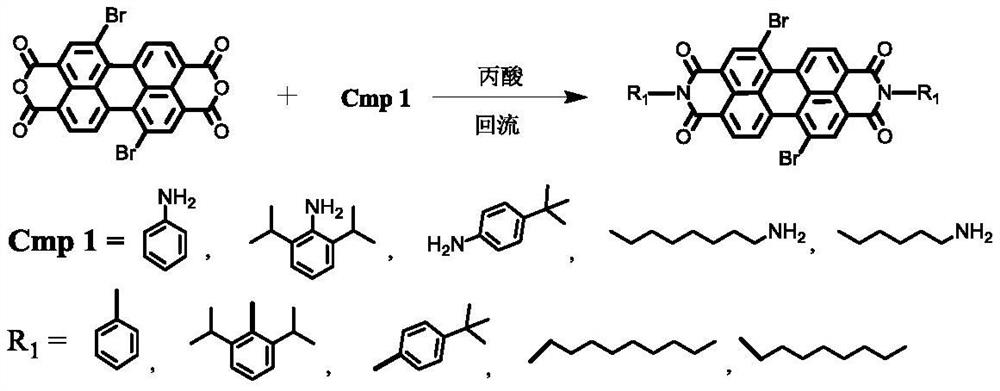
[0025] Under nitrogen protection, into a 100mL two-necked bottle, add 5.4g (9mmol) of the compound 1,7-dibromoperylenediimide, 3.6g (20mmol) of 2,6-diisopropylaniline, dissolve in 100ml In water propionic acid, react in an oil bath at 120-125°C for 24 hours. The progress of the reaction was detected by thin-layer chromatography. After the reaction was completed, it was cooled to room temperature, the reaction solution was poured into 80 ml of water, filtered under reduced pressure, and recrystallized from ethanol to obtain compound PDI-Br, 7.8 g, yield: 92%. 1 H NMR (300MHz, CDCl 3 )δ9.21(s,2H),8.82(d,J=6Hz,6H),7.68-7.54(m,J=7.7Hz,6H),5.63(m,2H),1.38(d,J=9.0Hz ,12H).
[0026] step 2
[0027]
[0028] Under the protection of nitrogen, the compound PDI-Br (6g, 7mmol), carbazole (2g, 7mmol), o-phenanthroline (1.2g, 7mmol), potassium carbonate (1g, 7mmol), and catalytic amount of potassium iodide were dissolved in 100ml DMF In the process, nitr...
Embodiment 2
[0033] step 1
[0034]
[0035] The synthesis of PDI-Be, yield 90%, 1 H NMR (300MHz, CDCl 3 ) δ9.16 (s, 2H), 8.78 (d, J=6Hz, 6H), 7.86-7.82 (m, 4H), 7.66-7.52 (m, 6H).
[0036] step 2
[0037]
[0038] The synthesis of PDI-K, yield 85%, 1 H NMR (300MHz, CDCl 3 ) 1 H NMR (300MHz, CDCl 3 )δ9.12(s,2H),8.76(d,J=6Hz,6H),7.86-7.82(m,4H),7.66-7.52(m,6H),7.48-7.36(m,8H),1.28( s, 6H).
[0039] step 3
[0040]
[0041] Synthesis of PDI-BD: Yield 72%, 1 H NMR (300MHz, CDCl 3 ) 1 H NMR (300MHz, CDCl 3 )δ9.14 (s, 2H), 8.78 (d, J=6Hz, 6H), 8.12-7.86 (m, 4H), 7.88-7.84 (m, 4H), 7.68-7.54 (m, 6H), 7.48- 7.36(m,8H), 1.28(s,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com