Preparation method of glycosylated hemoglobin calibration quality control product
A technology of glycosylated hemoglobin and hemoglobin, which is applied in the field of medical testing, can solve the problems of biological safety of blood products, unfavorable industrialization, and rare samples, and achieve stable and controllable methods, which are conducive to industrial production and easy to obtain.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
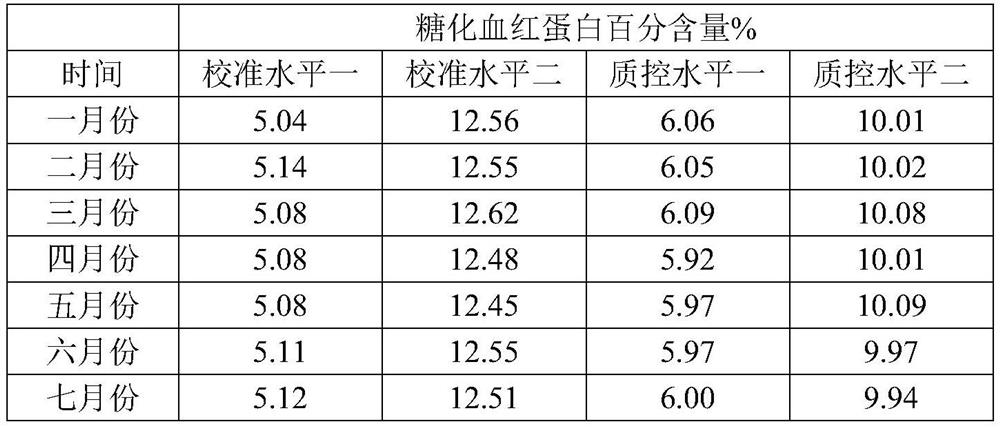
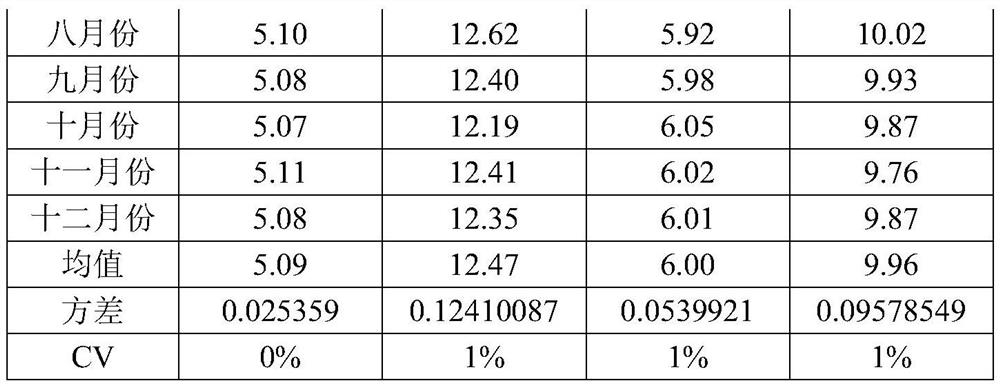
[0036] Embodiment 1 prepares hemoglobin calibration standard
[0037] S1. Pretreatment of pig blood samples: collect fresh pig blood samples, mix them with the cleaning solution at a ratio of 1:1, mix well, centrifuge, remove the supernatant, and repeat three times to obtain pig red blood cells. The components of the cleaning solution are as follows:
[0038] Sodium chloride NaCl: 9g / L
[0039] CAA: 1%
[0040] S2, preparation of porcine hemoglobin: freeze-thaw the red blood cells obtained in S1 once at -20°C, add 1-10 times the volume of purified water, stir slowly at 2-8°C for 4-8 hours, and centrifuge to take the supernatant , to obtain porcine hemoglobin;
[0041] S3, glycosylation reaction: add the hemoglobin obtained in S2 to the glycosylation solution at a ratio of 1:10, and place it at 37°C for 3 days, and the composition of the glycosylation solution is as follows:
[0042] HEPES: 20mM
[0044] CAA: 1%
[0045] Glucose: 50mM
[004...
Embodiment 2
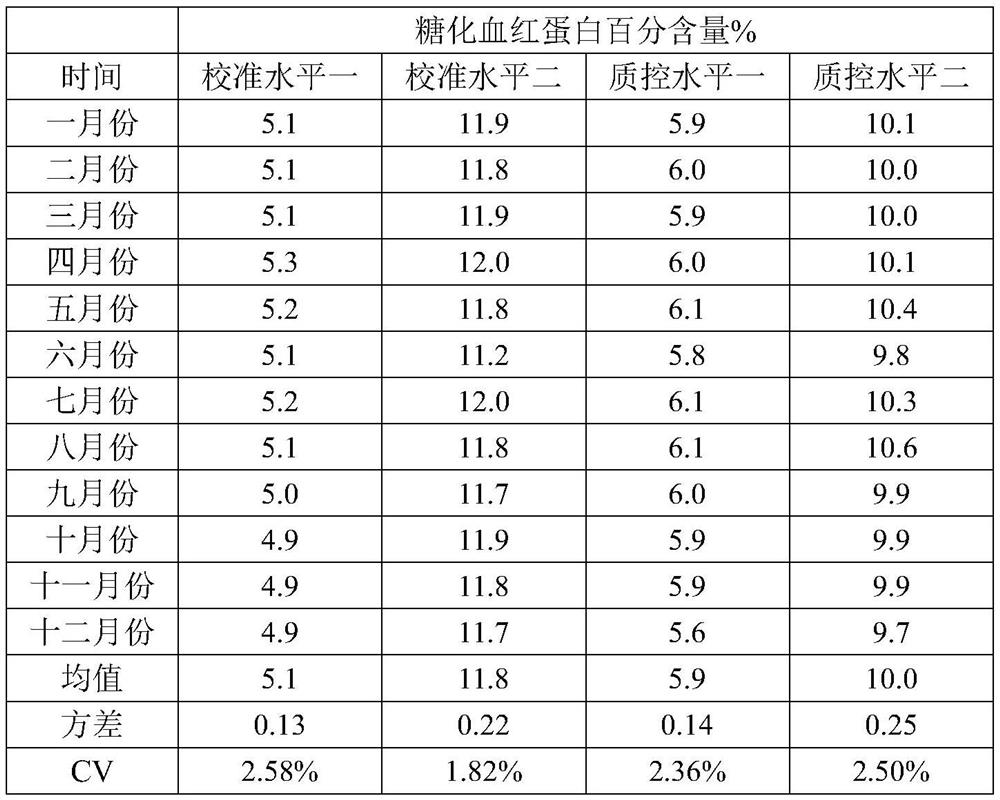
[0061] Embodiment 2 prepares hemoglobin calibration standard
[0062] The difference from Example 1 is that the mass concentration of the preservative CAA is 0.1%, 2 mM ethylenediaminetetraacetic acid (EDTA) is added to the glycosylation fluid and the dialysate, and the rest of the conditions and test methods are the same as in Example 1.
[0063] S1. Pretreatment of pig blood samples: collect fresh pig blood samples, mix them with the cleaning solution at a ratio of 1:1, mix well, centrifuge, remove the supernatant, and repeat three times to obtain pig red blood cells. The components of the cleaning solution are as follows:
[0064] NaCl: 9g / L
[0065] CAA: 0.1%
[0066] S2, preparation of porcine hemoglobin: freeze-thaw the red blood cells obtained in S1 once at -20°C, add 1-10 times the volume of purified water, stir slowly at 2-8°C for 4-8 hours, and centrifuge to take the supernatant , to obtain porcine hemoglobin;
[0067] S3, glycosylation reaction: add the hemoglobi...
Embodiment 3
[0088] The optimization of embodiment 3 cleaning liquid addition proportions
[0089] The difference from Example 1 is that the ratio of fresh pig blood sample to cleaning solution is 1:2, and other conditions and testing methods are the same as in Example 1.
[0090] S1. Pretreatment of pig blood samples: collect fresh pig blood samples, mix them with the cleaning solution at a ratio of 1:2, centrifuge after mixing, remove the supernatant, and repeat three times to obtain porcine red blood cells. The components of the cleaning solution are as follows:
[0091] NaCl: 9g / L
[0092] CAA: 1%
[0093] S2, preparation of porcine hemoglobin: freeze-thaw the red blood cells obtained in S1 once at -20°C, add 1-10 times the volume of purified water, stir slowly at 2-8°C for 4-8 hours, and centrifuge to take the supernatant , to obtain porcine hemoglobin;
[0094] S3, glycosylation reaction: add the hemoglobin obtained in S2 to the glycosylation solution at a ratio of 1:10, and place...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com