Preparation method of 17-formic acid steroid compound
A technology of steroidal compounds and compounds, applied in the field of preparation of 17-formic acid steroidal compounds, can solve the problems of low reaction temperature, unfriendly environment, increased production energy consumption, etc., and achieve mild reaction conditions and less alkali consumption , The effect of reducing the production of isomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] This specific embodiment proposes a preparation method of 17-formic acid steroid compound, which is characterized in that it comprises the following steps: dissolving compound 1 in an organic solvent, then adding a base, and then adding PyHBr 3 (i.e. pyridinium bromide salt) stirred at 20-30°C for 2-4h, continued to add sodium bisulfite to the reaction product to quench the reaction, then adjusted the pH to 2-3 with acid, and then distilled and recovered the organic solvent, followed by washing and drying to obtain the 17-formic acid steroid compound (ie compound 2); the structural formula of the compound 1 is The organic solvent is one or both of 1,4-dioxane and tetrahydrofuran; the alkali is one or more of sodium hydroxide, potassium hydroxide, sodium tert-butoxide and potassium tert-butoxide Kind; The compound 1 and the PyHBr 3 The mass ratio is (1-2):(1.5-3); the material ratio of the compound 1 to the organic solvent is 1g:10-20mL.
[0022] The reaction equation...
Embodiment 1
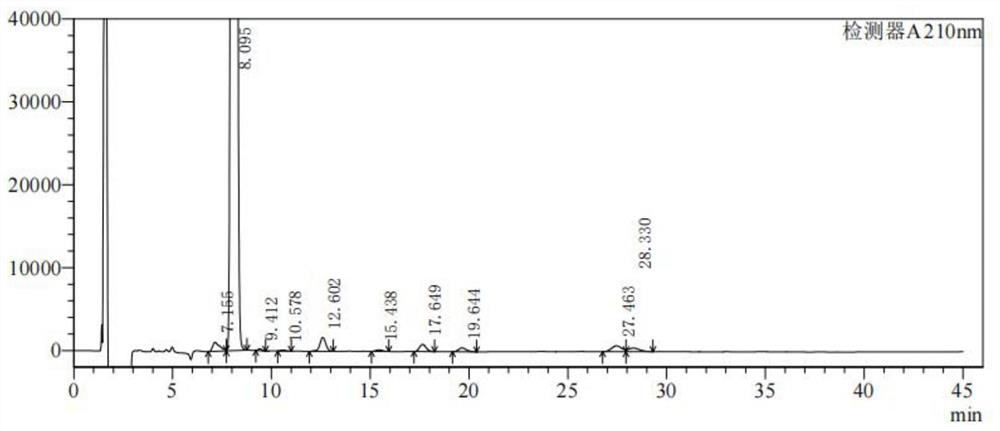
[0026] The present embodiment proposes a preparation method of 17-formic acid steroid compound, comprising the following steps: dissolving 5 g of compound 1 in 50 ml of 1,4-dioxane, adding 50 ml of 40% aqueous sodium hydroxide solution, and slowly adding in three times 7.5gPyHBr 3 , after stirring for 2 hours at 20°C, add 5ml of 15% sodium bisulfite to quench the reaction, adjust pH=2 with concentrated hydrochloric acid, recover 1,4-dioxane by atmospheric distillation, add 100ml of water, stir, filter, filter The cake was rinsed with water until it was neutral, and air-dried at 50°C to obtain compound 2 with a yield of 96.7%, a purity of 98.615%, and a recovery of 1,4-dioxane of 91.5%.
[0027] From figure 1 It can be seen that the 17-formic acid steroid compound was obtained with high purity.
Embodiment 2
[0029] The present embodiment proposes a kind of preparation method of 17-formic acid steroid compound, comprises the following steps:
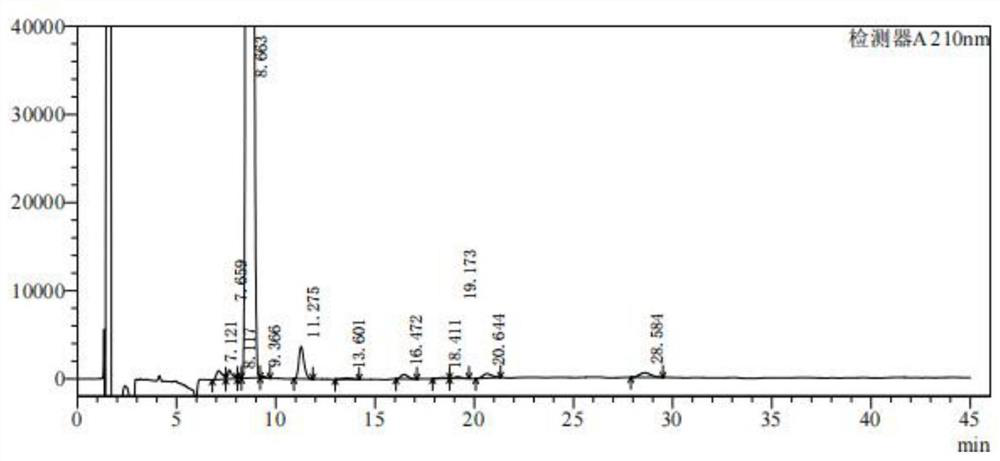
[0030] Dissolve 20g of compound 1 in 200ml of 1,4-dioxane, add 200ml of 45% potassium hydroxide aqueous solution, and slowly add 30g of PyHBr in three 3 , after stirring for 2 hours at 30°C, add 20ml of 15% sodium bisulfite to quench the reaction, adjust the pH to 2 with concentrated hydrochloric acid, recover 1,4-dioxane by atmospheric distillation, add 400ml of water, stir, filter, filter The cake was rinsed with water until it was neutral, and air-dried at 50°C to obtain Compound 2 with a yield of 96.3%, a purity of 98.252%, and a recovery of 1,4-dioxane of 92.8%.
[0031] From figure 2 It can be seen that the 17-formic acid steroid compound was obtained with high purity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com