Preparation method of multi-channel surface modified amorphous iron oxide nanospheres
A technology of surface modification and amorphous state, which is applied in the field of preparation of lithium-ion battery negative electrode and multi-channel surface modified amorphous Fe2O3 nanosphere material, can solve the problems of high experimental cost and complicated operation, and achieve high repeatability, The preparation process is simple and the effect of reducing structural stress/strain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] S1: Dissolve 0.226g of analytically pure potassium ferricyanide and 0.026g of ammonium dihydrogen phosphate into 45ml of deionized water, stir at room temperature at 550rmp for 10min, and obtain a clear liquid.
[0036]S2: Transfer the liquid obtained above to a polytetrafluoroethylene autoclave (total capacity: 100ml), and keep it at 180°C for 6h with a heating rate of 6°C / min, then wait for the reactant to cool down to room temperature naturally, take out mixture.
[0037] S3: The above mixture was centrifuged and washed three times with deionized water and absolute ethanol respectively, and the obtained powder was dried in a drying oven at 80°C for 10 hours to obtain multi-channel surface-modified amorphous Fe 2 o 3 nanosphere material.
Embodiment 2
[0039] S1: Dissolve 0.326g of analytically pure potassium ferricyanide and 0.043g of ammonium dihydrogen phosphate into 55ml of deionized water, stir at room temperature at 550rmp for 20min, and obtain a clear liquid.
[0040] S2: Transfer the liquid obtained above to a polytetrafluoroethylene autoclave (total capacity of 100ml), and keep it at 190°C for 6h with a heating rate of 6°C / min, then wait for the reactants to cool down to room temperature naturally, take out mixture.
[0041] S3: The above mixture was centrifuged and washed three times with deionized water and absolute ethanol respectively, and the obtained powder was dried in a drying oven at 100°C for 8 hours to obtain multi-channel surface-modified amorphous Fe 2 o 3 nanosphere material.
Embodiment 3
[0043] S1: Dissolve 0.376g of analytically pure potassium ferricyanide and 0.053g of ammonium dihydrogen phosphate into 65ml of deionized water, and stir at room temperature for 30min at 550rmp to obtain a clear liquid.
[0044] S2: Transfer the liquid obtained above to a polytetrafluoroethylene autoclave (total capacity: 100ml), and keep it at 200°C for 6h with a heating rate of 6°C / min, then wait for the reactant to cool down to room temperature naturally, and take it out mixture.
[0045] S3: The above mixture was centrifuged and washed three times with deionized water and absolute ethanol respectively, and the obtained powder was dried in a drying oven at 80°C for 12 hours to obtain multi-channel surface-modified amorphous Fe 2 o 3 nanosphere material.
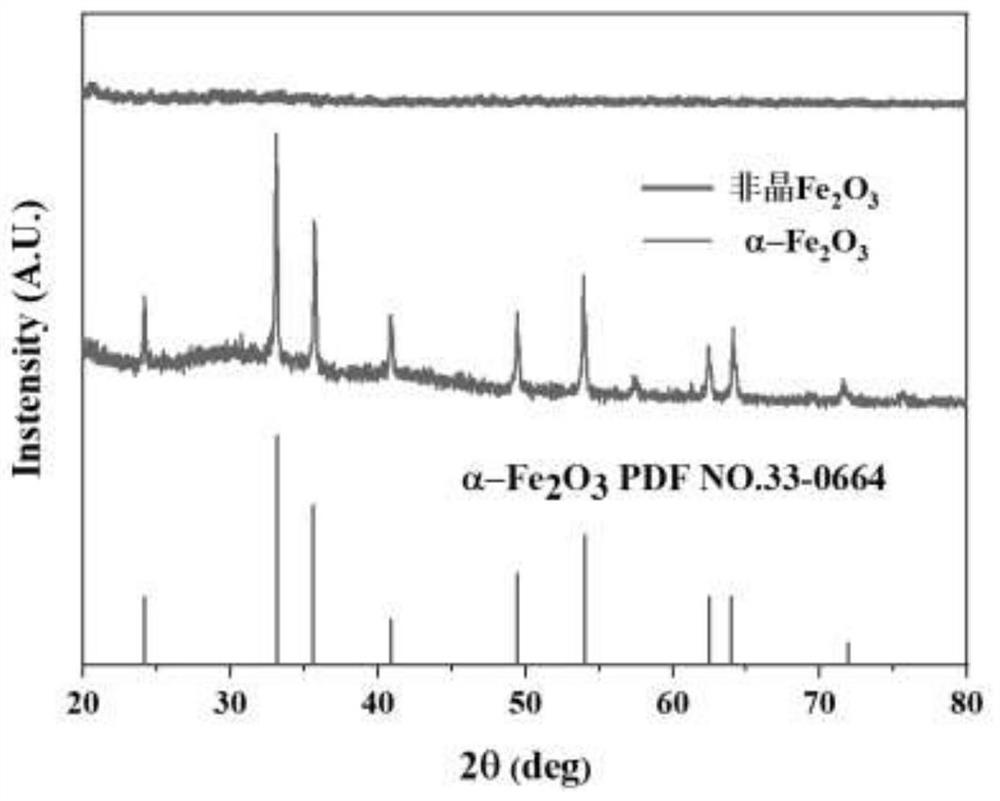
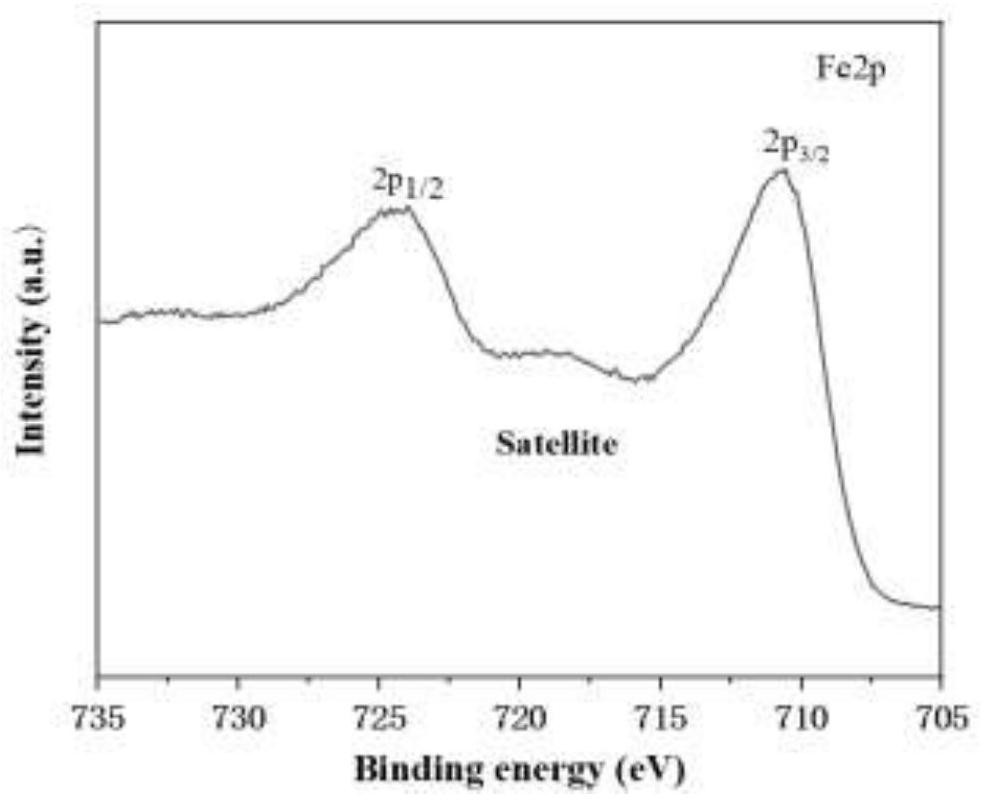
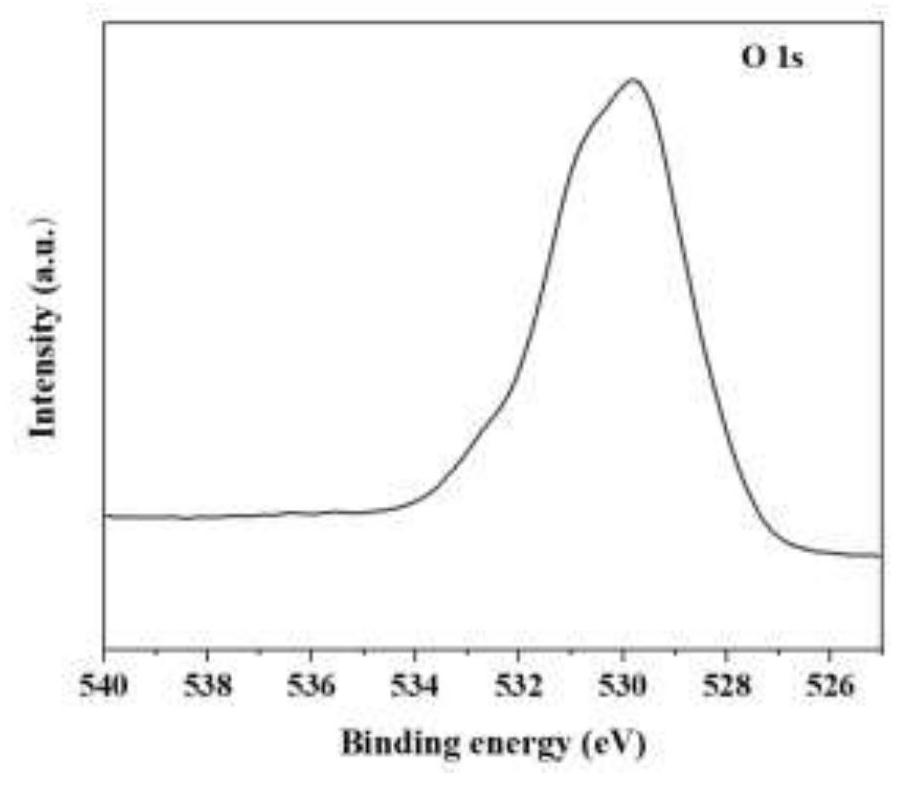
[0046] Such as figure 1 As shown, the multi-channel surface modified amorphous Fe obtained in Example 3 2 o 3 In the XRD pattern of the nanosphere material, there is no obvious diffraction peak in the XRD pattern of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com