Arginine deiminase producing strain and construction method thereof
A technology of arginine deiminase and construction method, applied in the field of arginine deiminase production bacteria and its construction, to achieve the effect of simple composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Codon optimization
[0038] The nucleotide sequence of the arcA gene obtained by the inventor from the existing database is shown in SEQ ID NO.1. In order to make the arcA gene more suitable for the expression system of Bacillus subtilis, the present invention optimizes the codons of the nucleotide sequence composition of the arcA gene.
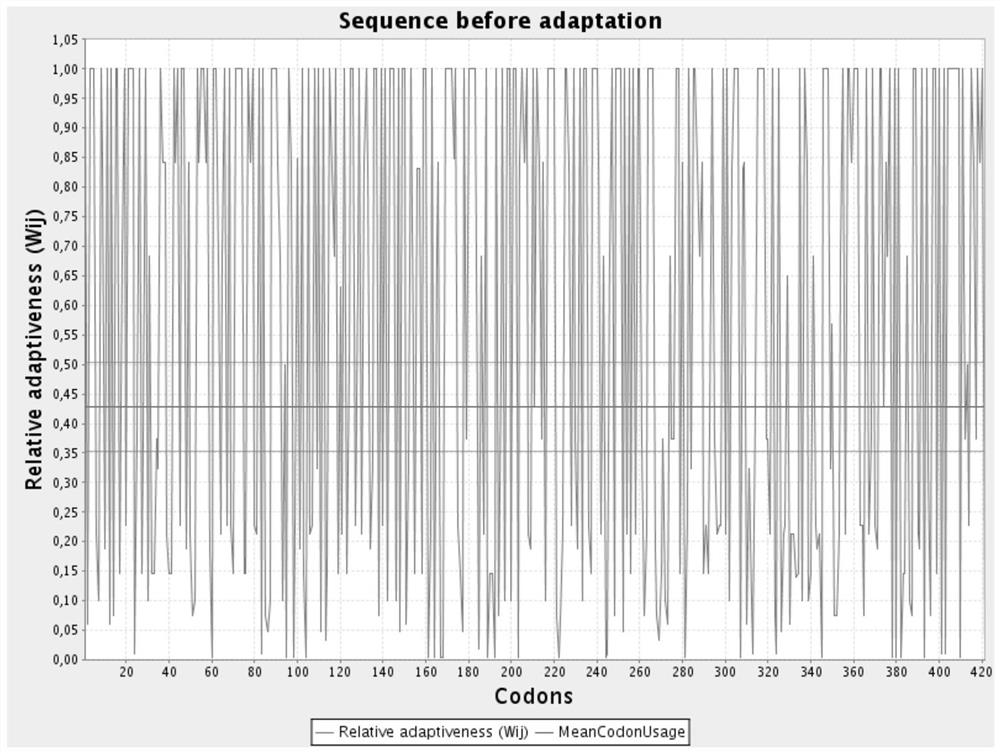
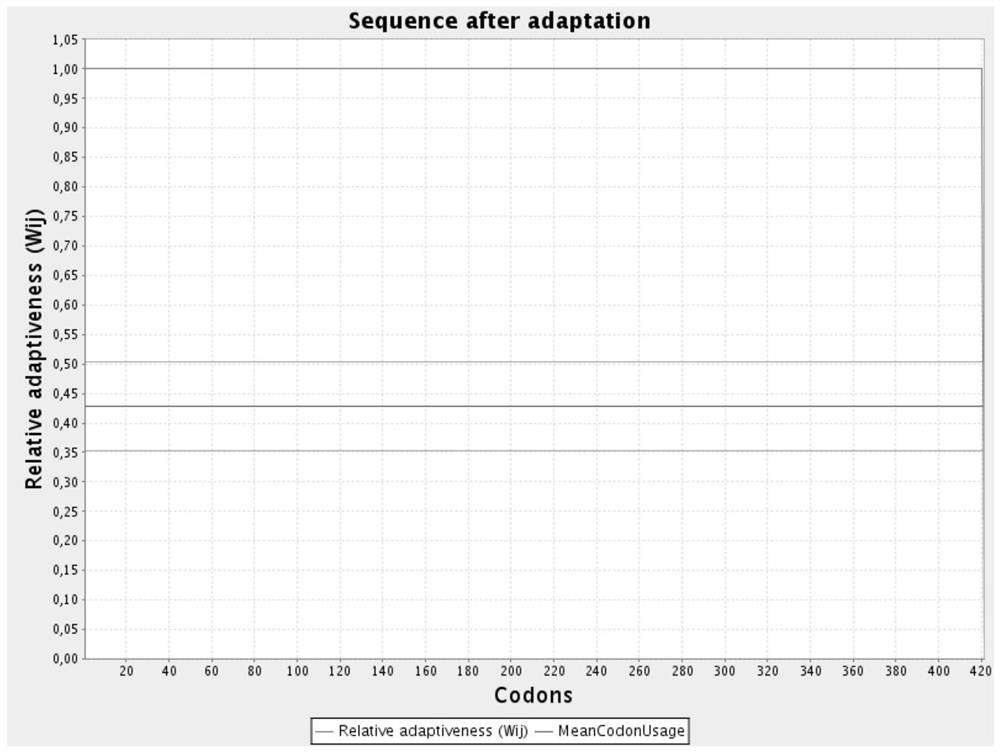
[0039] The nucleotide sequence of the arcA gene after codon optimization is shown in SEQ ID NO.2; the codon relative fitness figure before optimization is shown in figure 1 Shown; the codon relative fitness map after optimization is shown in figure 2 shown.
[0040] Depend on figure 2 It can be seen that the codon relative fitness of the arcA gene after codon optimization can reach 1.0, which significantly improves the adaptability of the protein-coding gene in the Bacillus subtilis expression system.
Embodiment 2
[0041] Embodiment 2: Construction of recombinant expression vector
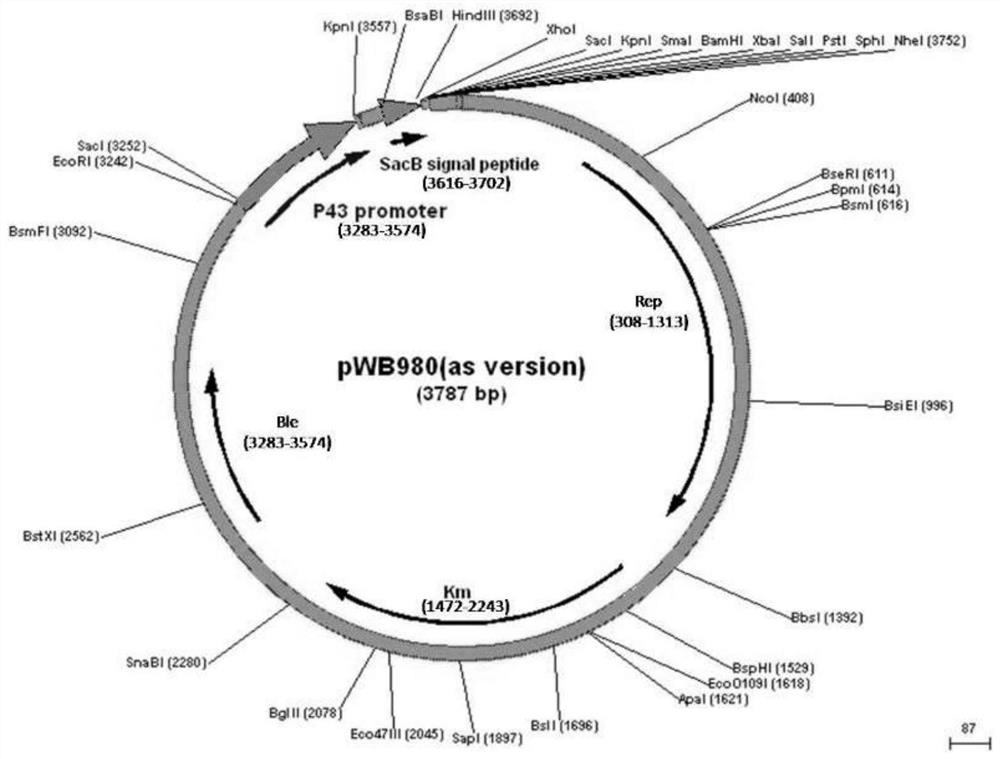
[0042] Plasmid pWB980 ( image 3 shown) treated with EcoRI and KpnⅠ double enzyme digestion ( Figure 4 ), the Pg3 strong promoter sequence (shown in SEQ ID NO.3) was integrated into the plasmid pWB980 after the double enzyme digestion treatment to obtain the plasmid pWB980-Pg3 ( Figure 5), its nucleotide sequence is shown in SEQ ID NO.4.
[0043] The plasmid pWB980-Pg3 was digested with BamHI and SphⅠ, and the codon-optimized arcA gene (shown in SEQ ID NO.2) was integrated into the digested plasmid pWB980-Pg3 ( Image 6 ) to obtain the recombinant expression vector (pWB980-Pg3-arcA).
[0044] The constructed recombinant expression vector was digested with two enzymes BamHI and SphⅠ, and the results were as follows: Figure 7 shown. The results showed that the arcA gene (shown in SEQ ID NO.2) had been successfully integrated into the plasmid pWB980-Pg3.
Embodiment 3
[0045] Embodiment 3: Construction of arginine deiminase-producing bacteria
[0046] The recombinant expression vector (pWB980-Pg3-arcA) constructed in Example 2 was introduced into Bacillus subtilis 168 to obtain a transformant. Spread the transformant on an LB plate containing 30 μg / ml kanamycin (kan), pick out a single colony capable of growing, and use it as a positive transformant.
[0047] Inoculate positive transformants into glycerol agar medium containing 30 μg / ml kanamycin and culture at 37°C until OD 600 =0.6, slowly lower the temperature to 28°C, add IPTG (to make the final concentration of IPTG 0.2mmol / L), and induce culture for 16h. After the induction culture was completed, the bacteria were disrupted by ultrasonication, centrifuged, and the supernatant was separated, and detected by Western bolt. The results were as follows: Figure 8 shown. There is an expression band at 50.4KDa, which is consistent with the theoretically calculated molecular weight of the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com