Amide group modified hyper-crosslinked adsorption resin as well as preparation method and application thereof
A technology of ultra-high cross-linking and adsorption resin, applied in the direction of selective adsorption, chemical instruments and methods, ion exchange, etc., can solve the problems of complex synthesis route of cross-linking adsorption resin, and achieve the effect of high adsorption selectivity and high-efficiency separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A preparation method of amide group modified ultra-high cross-linked adsorption resin, comprising the following steps:
[0039] First, add the hydrophilic network monomer N,N'-(4,4'-methylenediphenyl) bismaleimide and the hydrophobic network monomer di Vinylbenzene, and an appropriate amount of initiator azobisisobutyronitrile; then add 100mL dimethylformamide into the three-necked flask, stir and dissolve at room temperature, and then heat up to 80°C after bubbling nitrogen for 0.5h, and keep the reaction for 24h. get the reaction product;
[0040]Then, the above reaction product was Soxhlet-extracted with tetrahydrofuran for 24 hours, rinsed and soaked in water for 12 hours until odorless; finally, vacuum-dried at 80°C for 24 hours to obtain an amide group-modified ultra-high cross-linked adsorption resin. Its yield was 99.3%.
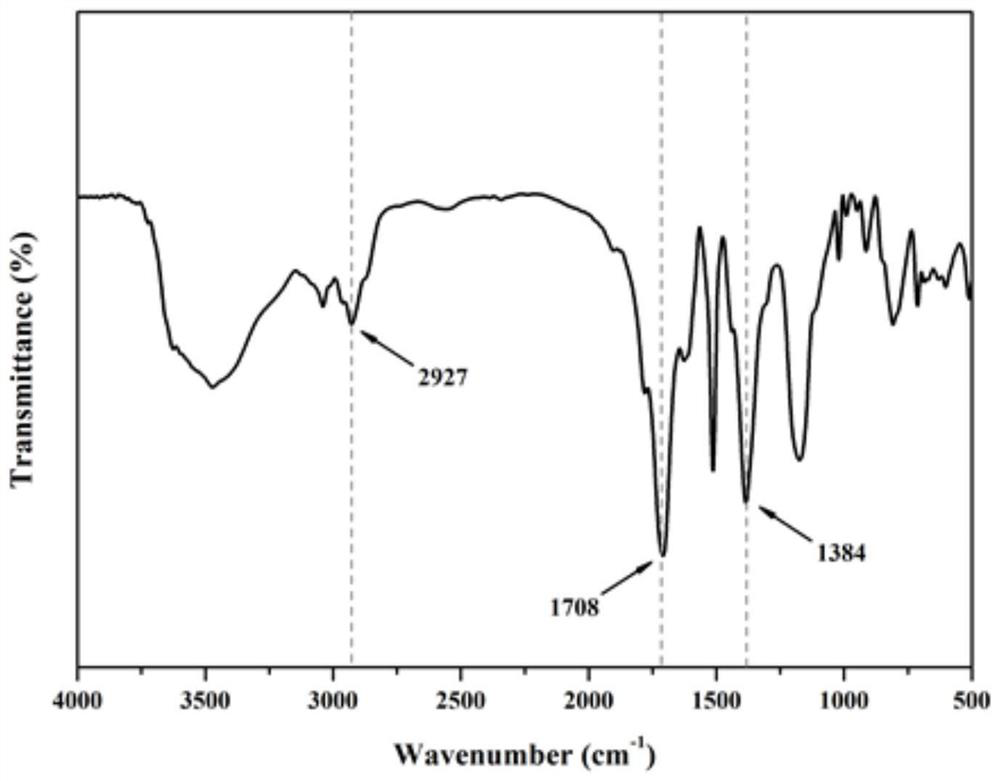
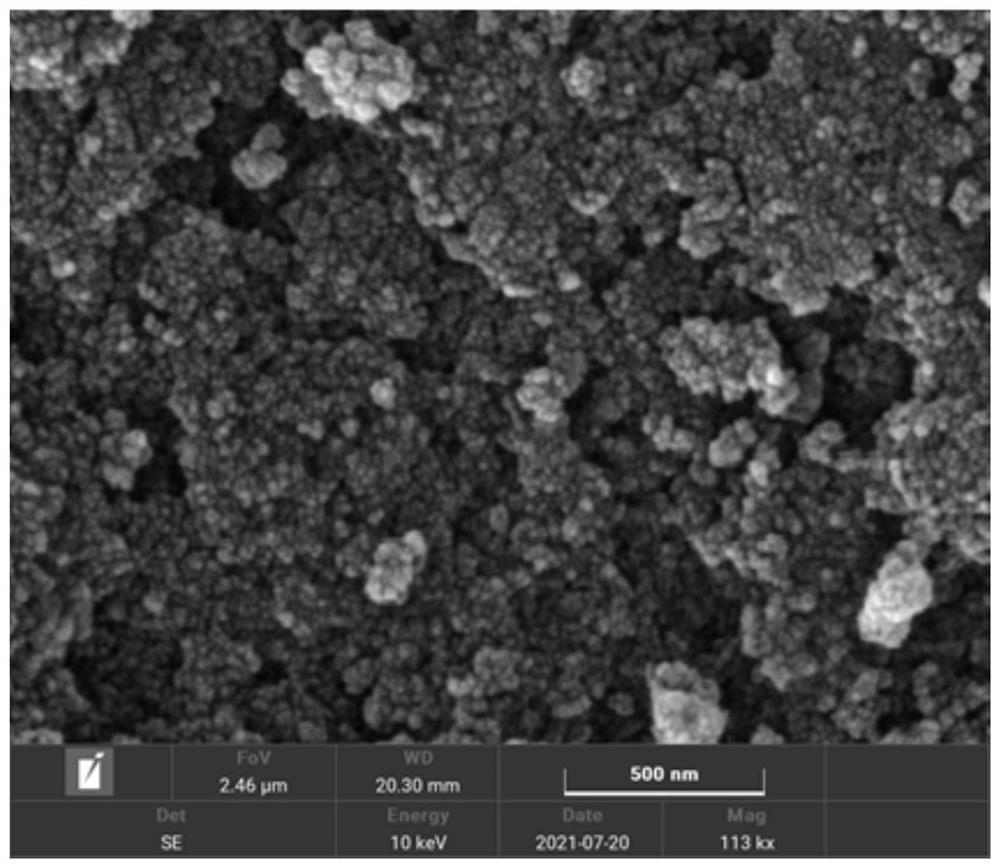
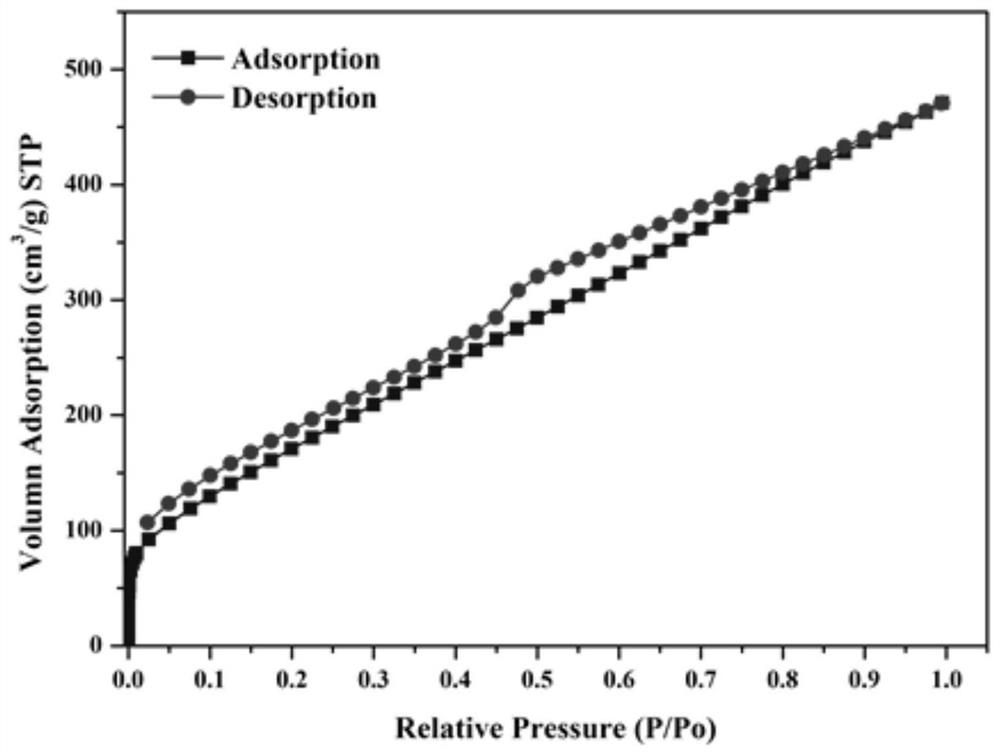
[0041] The specific surface area of the amide group-modified ultra-high cross-linked adsorption resin prepared by the above preparation m...
Embodiment 2
[0043] In Example 2, the molar ratio of the hydrophilic network monomer to the hydrophobic network monomer was 1:1.5, and the others were the same as in Example 1; the yield of the obtained amide group-modified ultra-high cross-linked adsorption resin was 85.6%.
Embodiment 3
[0044] In Example 3, the molar ratio of the hydrophilic network monomer to the hydrophobic network monomer was 1:0.33, and the others were the same as in Example 1; the yield of the obtained amide group-modified ultra-high cross-linked adsorption resin was 76.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap