Chondrosulphatase B as well as coding gene and construction method thereof
A chondroitin sulfate enzyme and gene technology, applied in the field of genetic engineering, can solve problems such as limitations, limited thermostability, pH stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 In vitro expression of chondroitinase B
[0033] With primer F (5'-CGC GGATCC GCGGAATTCCTGGTGCACAACCAGC-3', BamHI site underlined, SEQ ID NO: 3) and primer R (5'-CCG CTCGAG ATTCGTGACTTCGTTATTGGCGAGG-3', XhoI site underlined, SEQ ID NO: 4), the chondroitinase B gene was amplified from the genome of Microbulbifer sp. The amplicon is 2181 bp in length and encodes mature chondroitinase B, excluding the signal peptide.
[0034] The PCR reaction system was as follows: 5 μL of 10×Taq DNA polymerase buffer, 4 μL of 10 mmol / L 4 kinds of dNTPs mixture, 1 μL of gene-specific upstream primers, 1 μL of gene-specific downstream primers, 0.5 μL of Primer STAR enzyme, and 2 μL of genome template , add sterile water to make up to 50 μL.
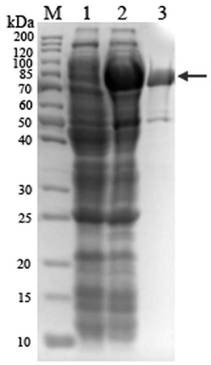
[0035] The PCR amplification program is: pre-denaturation (95°C, 5min); denaturation (94°C, 55s), annealing (55°C, 45s), extension (72°C, 90s), 30 cycles; extension (72°C, 10min) . The obtained PCR amplificat...
Embodiment 2
[0039] Example 2 Determination of the activity of chondroitinase B
[0040] Utilize 50mmol / L Na 2 HPO 4 -NaH 2 PO 42 mg / mL chondroitin sulfate B (Soiarbio, China) substrate solution was prepared in buffer (pH 8.0). Take 680 μL of substrate solution, add 15 μL of purified recombinant enzyme solution (0.6 mg / mL), react at 40° C. for 20 min, and stop the reaction in a boiling water bath for 10 min. After cooling the mixture to room temperature, the UV absorbance was measured at 232 nm. 1 unit of enzyme activity is defined as the amount of enzyme required to produce 1 μmoL of unsaturated carbon bonds per minute under the above conditions.
Embodiment 3
[0041] The enzymatic property of embodiment 3 chondroitin sulfate enzyme B
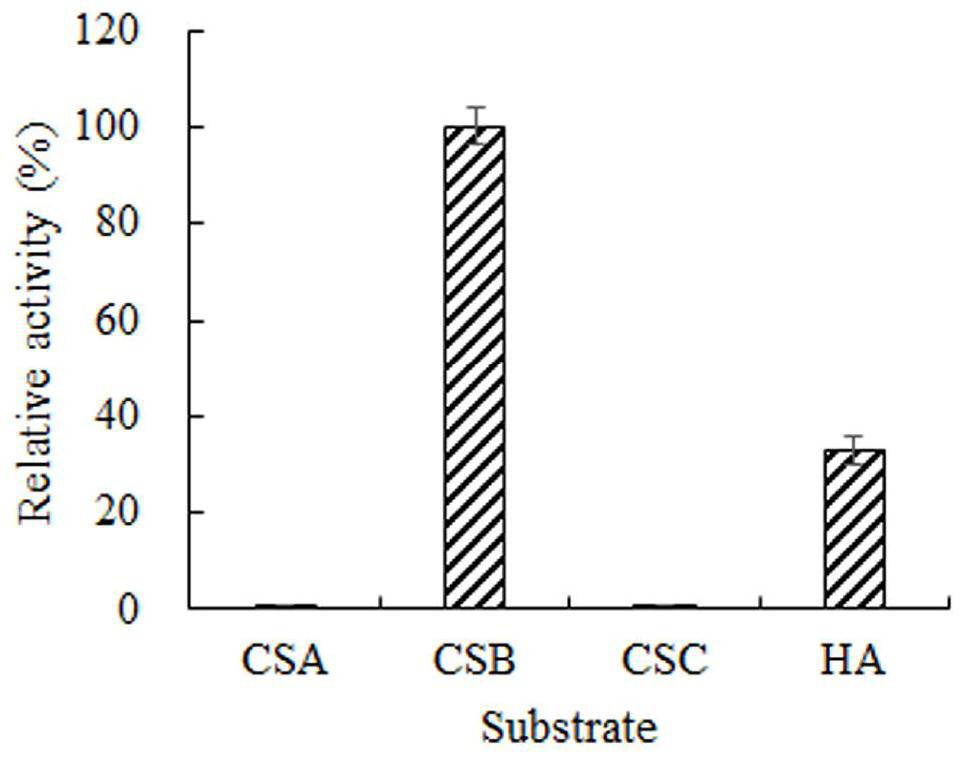
[0042] (1) Substrate specificity of chondroitinase B
[0043] Using the activity assay method of Example 2, measure the enzymatic activity using different substrates respectively, including chondroitin sulfate A (CSA), chondroitin sulfate B (CSB), chondroitin sulfate C (CSC) and hyaluronic acid (HA) , to study the substrate specificity of enzymes. The result is as figure 2 As shown, the recombinant enzyme is active on CSB and HA, indicating that the enzyme belongs to chondroitinase B.
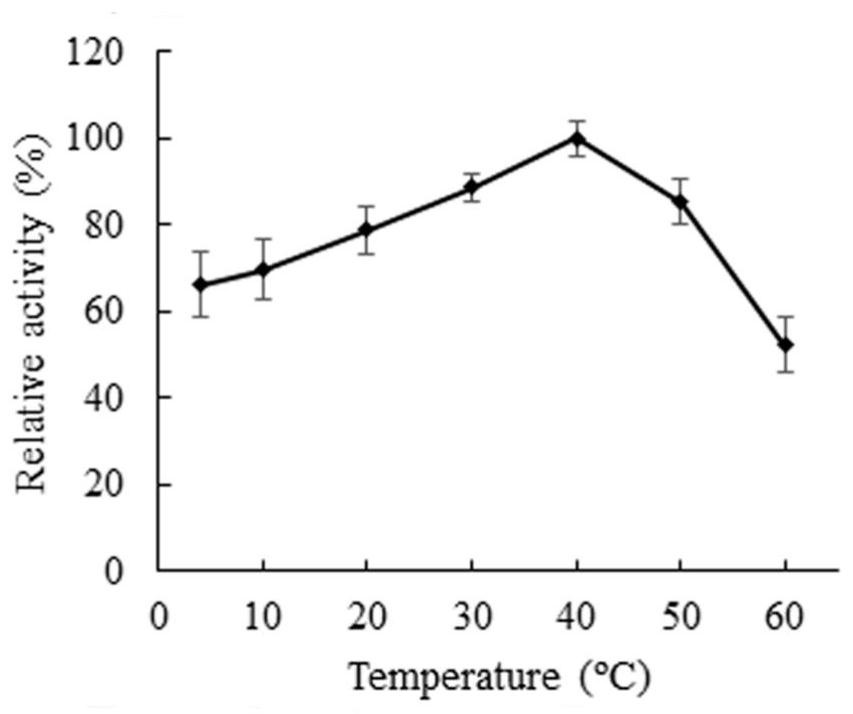
[0044] (2) Effects of temperature and pH on the activity and stability of chondroitinase B
[0045] The optimal reaction temperature of chondroitin sulfate B is determined in the range of 4-60°C, specific operation: adopt the method for measuring the activity of Example 2, under the conditions of 4, 10, 20, 30, 40, 50 and 60°C respectively reaction to measure enzyme activity. The result is as image 3 As shown, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com