Complement factor B inhibitor as well as pharmaceutical composition, preparation method and application thereof
A compound and solvate technology, applied in the field of complement factor B inhibitors and their pharmaceutical compositions, can solve the problems of heavy burden on patients, no specific therapeutic drugs, and difficult to cure with diversity, and achieve good regulation/inhibition effect, performance good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
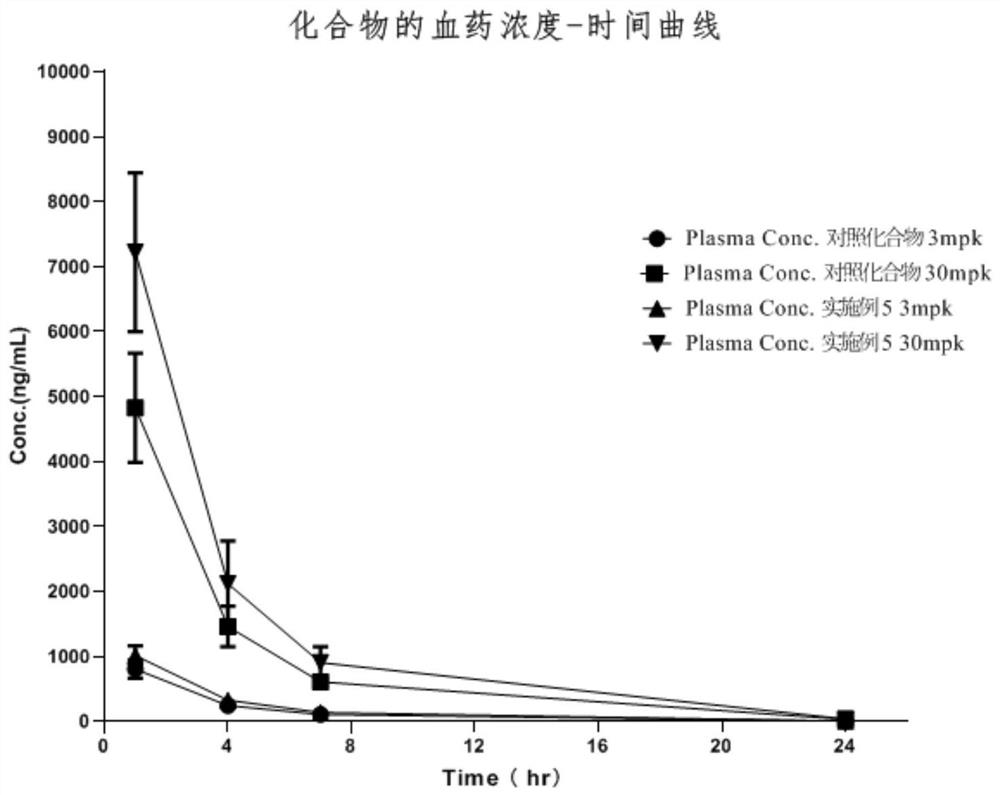
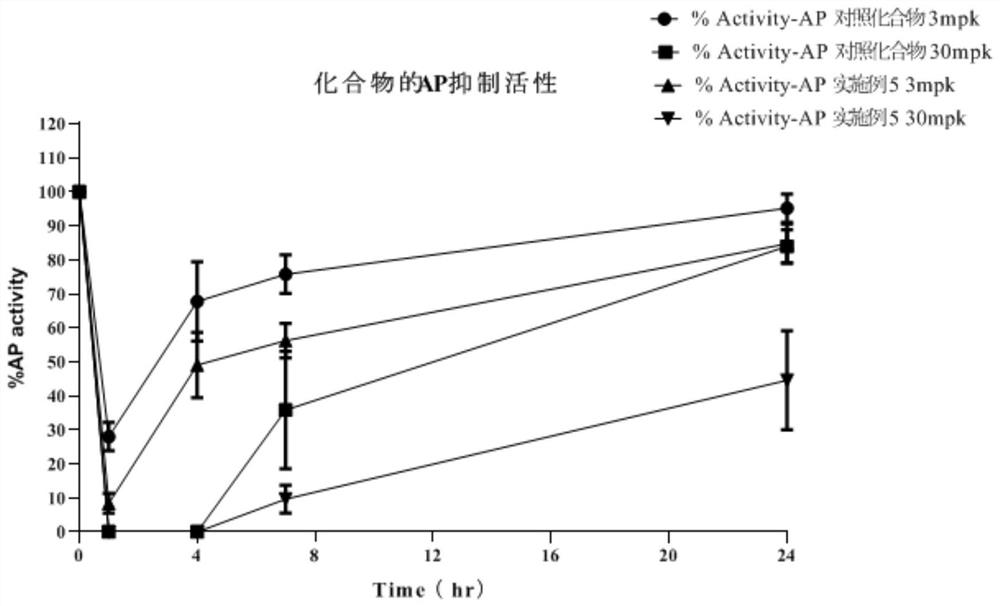
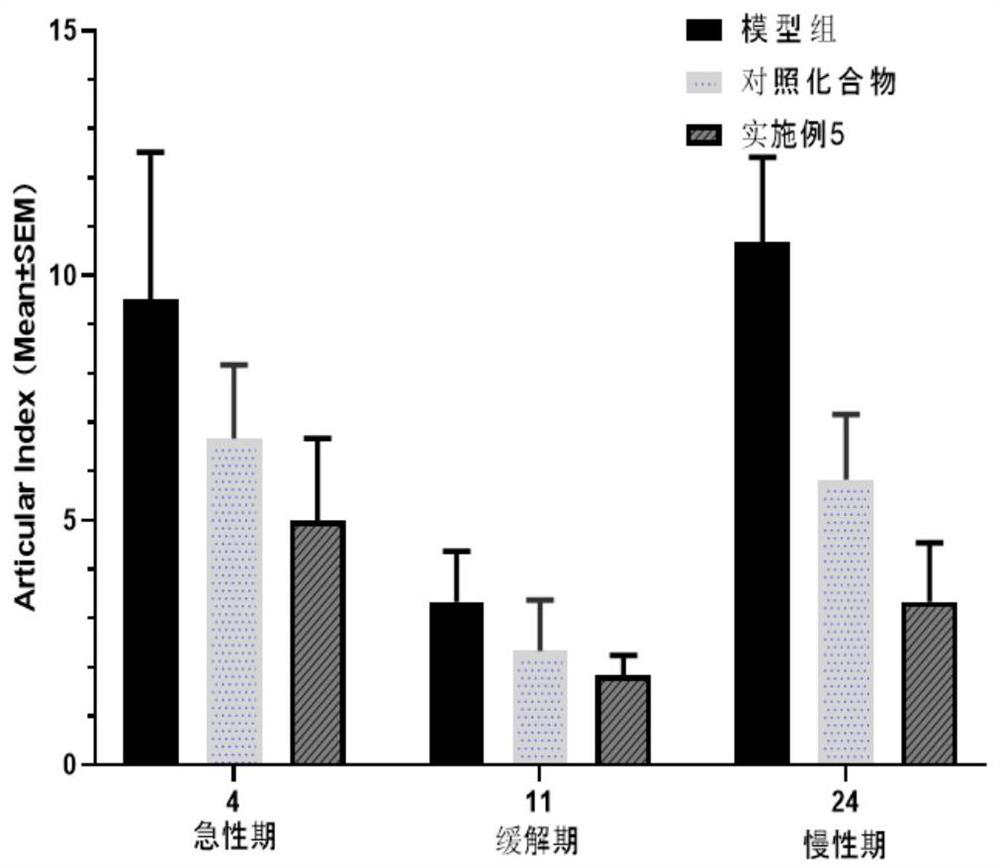
Image
Examples
Embodiment 1
[0176] Intermediate 1:
[0177]
[0178] In a 3L three-necked flask, sequentially add tetrahydrofuran (150mL) and 4-bromoxynil (50g), and slowly add isopropylmagnesium chloride lithium chloride complex (1.3M, 210mL) to the reaction system under nitrogen protection , the reaction was carried out at room temperature for 2 hours. Then the reaction system was diluted by adding anhydrous tetrahydrofuran (500mL) and cooled to -5°C, 4-methoxypyridine (25mL) was added, and benzyl chloroformate (35mL) was slowly added dropwise (maintaining the system temperature below 0°C), After the addition was complete, the reaction was stirred at 0°C for 2 hours, then warmed to room temperature and continued for 16 hours at room temperature. After the reaction, add 6M hydrochloric acid (150mL) and stir for half an hour and dilute with water (1000mL), extract twice with ethyl acetate (500mL), wash the combined extracts with saturated brine (50mL), and dry over anhydrous sodium sulfate And filte...
Embodiment 2
[0214] Intermediate 1:
[0215]
[0216] In a 250mL single-necked bottle, add dichloromethane (50mL), 5-methoxy-7-methyl-1H-indole (3g), Boc anhydride (5.68g), 4-dimethylaminopyridine (227mg) in sequence and triethylamine (2.26 g), the reaction was carried out at room temperature for 16 hours. After the reaction, the reaction solution was quenched by adding saturated ammonium chloride solution (5 mL), extracted three times with dichloromethane (20 mL), the combined organic phase was washed with water (5 mL), dried over anhydrous sodium sulfate and filtered, and the filtrate was concentrated. The residue was purified by column chromatography (petroleum ether:ethyl acetate=10:1) to obtain Intermediate 1 (4.6 g, yield: 94%). MS m / z(ESI):262.0[M+1].
[0217] Intermediate 2:
[0218]
[0219] In a 250 mL single-necked bottle, dichloromethane (80 mL), N-methylformanilide (3.8 g) and oxalyl chloride (3.6 g) were added in sequence, and the reaction was stirred at room tempera...
Embodiment 3
[0221] Intermediate 1:
[0222]
[0223] In a 100mL single-necked bottle, add 1-(vinyloxy)butane (10mL), triethylamine (300mg), o-phenanthroline (54mg), palladium acetate (67mg) and benzyl (2S, 4S)- 2-(4-cyanophenyl)-4-hydroxypiperidine-1-carboxylate (Example 1, Intermediate 5) (500 mg), the reaction mixture was heated to 90 °C under nitrogen protection and at this temperature Stir for 16 hours. After the reaction, the reaction solution was directly concentrated under reduced pressure, and the residue was purified by column chromatography (petroleum ether: ethyl acetate = 1:1) to obtain Intermediate 1 (360 mg, yield: 63%). MS m / z(ESI):384.8[M+23].
[0224] Intermediate 2:
[0225]
[0226] Under ice bath and nitrogen protection, a solution of trifluoroacetic acid (228mg) in dichloromethane (2mL) was added to a solution of diethylzinc (1M, 2mL) in dichloromethane (4mL); the reaction was carried out under ice bath After one hour, the reaction system was added dichlorom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com