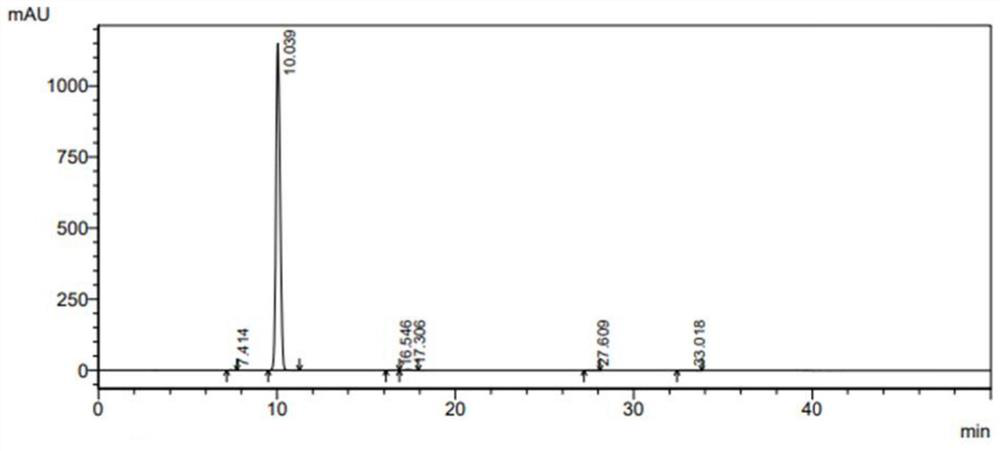
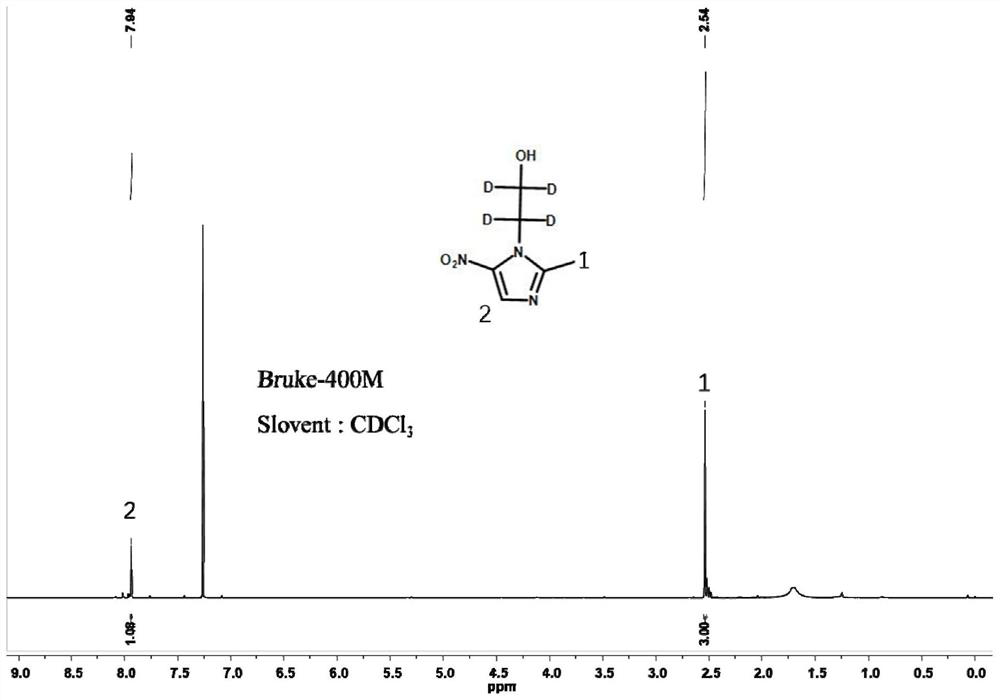
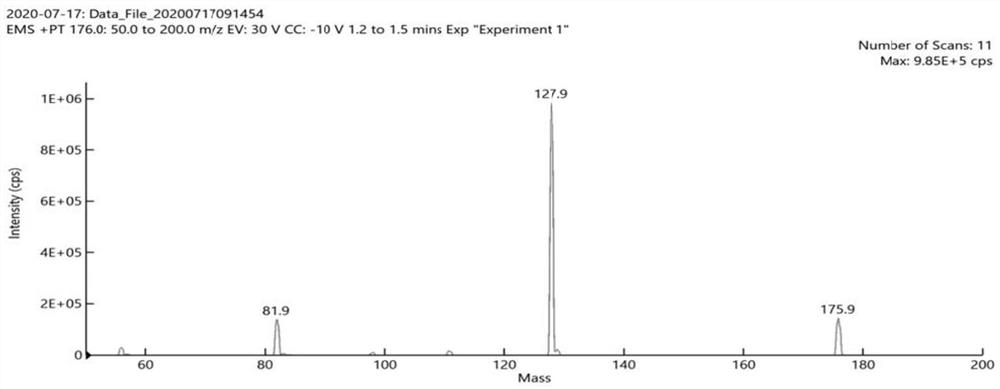
Stable isotope labeled metronidazole and synthesis method thereof
A stable isotope and synthetic method technology, applied in the field of chemical synthesis, can solve the problems of long synthetic process route, limited application, strict experimental conditions, etc., and achieve the effects of short process route, high use value, and easy separation and purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The synthesis process of stable isotope-labeled metronidazole is as follows:
[0034] S1. Weigh 15g of 2-bromoethanol-D4 into a 100mL three-neck flask, place it in an oil bath, set up a cooling collection device, and place the cold trap at -78°C. Insert the nitrogen needle below the liquid level and pass nitrogen protection. Then use a constant pressure dropping funnel to slowly drop 45mL of 25% NaOH solution into the solution, and control the drop to finish in about half an hour. After the drop, the temperature was raised to 100°C and kept for half an hour. The isotope-labeled oxirane was collected in the cold trap and immediately used for subsequent synthesis.
[0035] S2, when preparing the raw material for the first step, weigh 3.3 g of the raw material imidazole, put it into the reaction tube, add 8 mL of the mixed solution of formic acid and sulfuric acid (formic acid:concentrated sulfuric acid=8:1, volume ratio), Heat to 80°C to dissolve all imidazole as a raw ...
Embodiment 2
[0039] The synthesis process of stable isotope-labeled metronidazole is as follows:
[0040] S1. Weigh 15g of 2-bromoethanol-D4 into a 100mL three-neck flask, and place it in an oil bath; set up a cooling collection device, and place the cold trap at -70°C. Insert the nitrogen needle below the liquid level and start bubbling. Then use a constant pressure dropping funnel to add 45mL of 30% NaOH solution drop by drop to the solution at 1 drop per second, and control the drop to finish in about 40min. After dripping, start to heat up to 90°C and keep it for half an hour. Isotope-labeled oxirane is collected in the cold trap and used for subsequent synthesis immediately;
[0041] S2, when preparing the raw material for the first step, weigh 3.3 g of the raw material imidazole, put it into the reaction tube, add 8 mL of the mixed solution of formic acid and sulfuric acid (formic acid:concentrated sulfuric acid=7:1, volume ratio), Heat to 80°C to dissolve all imidazole as a raw mate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com