Method of treating cancer
A prostate cancer, dosage technology, applied in pharmaceutical formulations, medical preparations containing active ingredients, antitumor drugs, etc., can solve problems such as unmet medical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
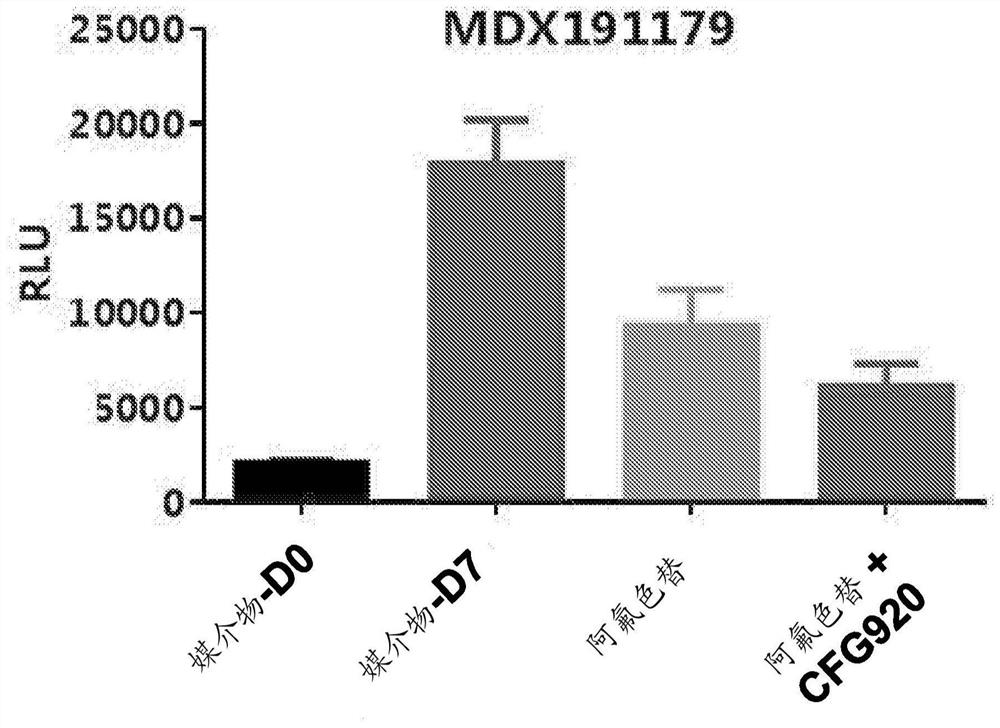
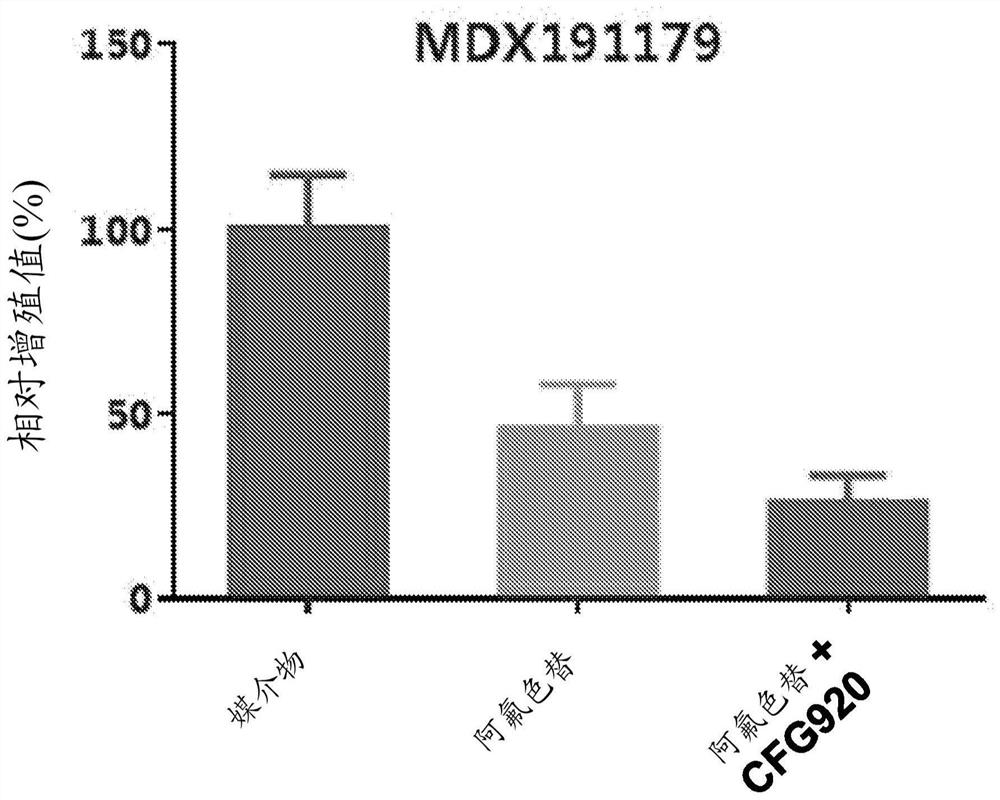
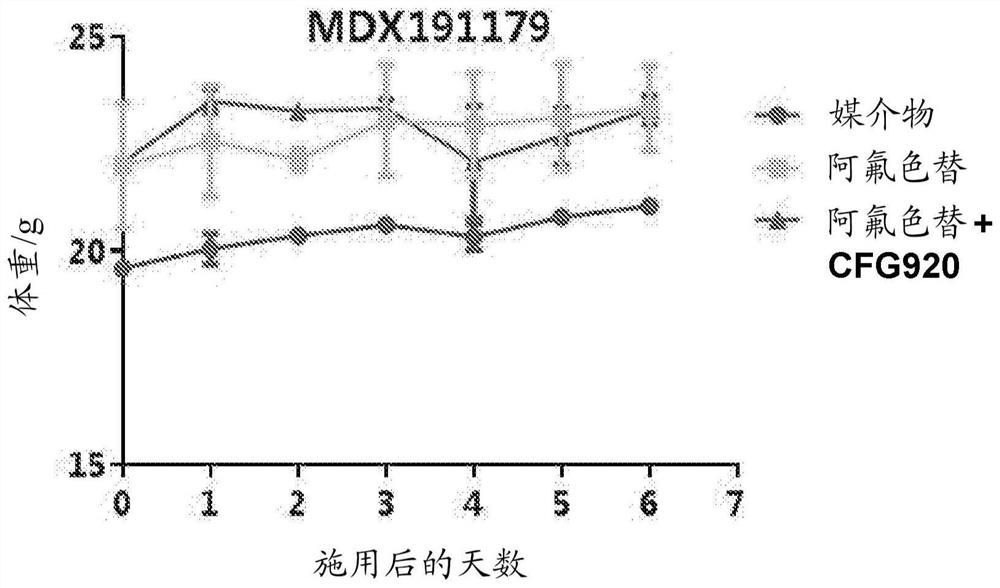
[0153] Example A: Mouse Mini-PDX Model Research
[0154] General Research Design
[0155] The goal of this study was to evaluate the in vivo therapeutic efficacy of CFG920 and afluoroxetine in the MDX191210 Mini-PDX model. The tumor sample was obtained from a 63-year-old male patient diagnosed with abiraterone-resistant prostate cancer.
[0156] animal
[0157] Male Balb / c nude mice were purchased from Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China, SCXK(Su) 2018-0008), certificate: 201806774. Species: House mouse (MusMusculus;); Strain: Balb / c naked; Age: 6 to 8 weeks; Sex: male; Body weight: 20 g to 25 g; Number of animals: 6 mice. Animals had free access to radiation sterilized dry pelleted chow throughout the study period. Animals had free access to sterile drinking water.
[0158] Mice were housed two animals per cage in a specific pathogen-free room at constant temperature and humidity. Captive conditions: temperature: 20°C-26°C; humidi...
Embodiment B
[0179] Example B: Dose Escalation and Efficacy Study of CFG920 and Prednisone Plus Afluoroxetine in Patients with Metastatic Castration-Resistant Prostate Cancer Following Standard of Care Therapy
[0180] overall design
[0181] The Phase I portion of the study was in patients who progressed or were intolerant to any anti-androgen therapy, such as abiraterone, enzalutamide, apalutamide, or any other later approved androgen receptor (AR) antagonist. CFG920 and A dose escalation study of the recommended phase II dose (RP2D) of combination therapy with prednisone + afluxetide. The Phase II portion of the study was initiated after progression or intolerance to any of two prior antiandrogen therapies (as described above) or one of more antiandrogen therapies plus a chemotherapeutic drug selected from docetaxel and cabazitaxel. The preliminary efficacy and safety of the combination therapy of CFG920 and prednisone + afluxerti compared to afuxerbin monotherapy were evaluated in mC...
Embodiment C
[0301] Example C: Summary of the Efficacy and Safety of Combination Therapy of CFG920+Afuroxerti in Human Patients
[0302] One of the objectives of the study in Example B was to evaluate patients in Cohort 1 who received a combination therapy dose of CFG920 75 mg + Prednisone 5 mg BID + Afluxetide 100 mg QD. The patient exhibited anticancer efficacy as assessed by prostate specific antigen (PSA) after receiving the combination therapy of CFG920 + prednisone + afluxerti treatment. The patient's clinical presentation is summarized below in support of this patent application.
[0303] Medical history: This study involved a 79-year-old Caucasian man with a past medical history of atrial fibrillation, hypertension, acid reflux, insomnia, and glaucoma; Lisinopril / Metoprolol, Aluminum Hydroxide / Simethicone, Temazepam, Timolol / Travoprost. He was diagnosed with prostate cancer at the age of 66. She underwent radical prostatectomy shortly after her prostate cancer diagnosis, and des...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com