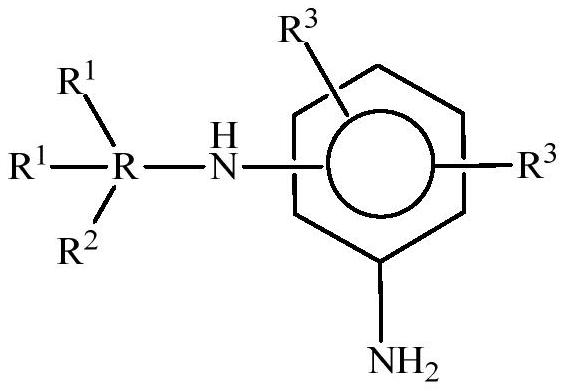
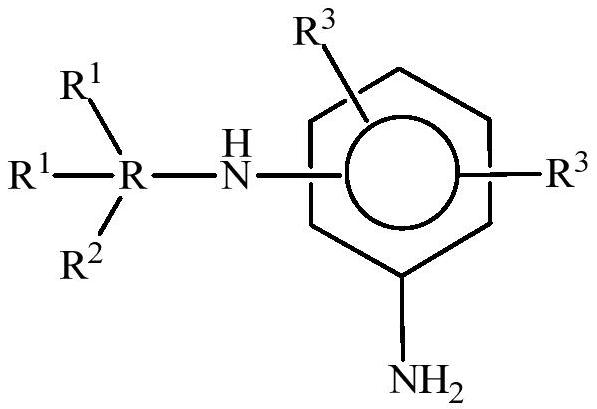
Polyethers and their use in the production of flexible polyurethane foams
A technology of polyether and polyether polyol, which is used in the preparation of polymer polyol compositions for flexible polyurethane foams and in the field of flexible polyurethane foams, and can solve problems such as insufficient VOC release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0120] The preparation of free rise foams generally requires mixing all components together except the isocyanate component, then adding the isocyanate component to the mixture and briefly mixing. The mixture is then poured into boxes and left to rise freely. The settling of the foam is measured and the foam is oven cured eg at 125°C for 5 minutes. After 16 hours at room temperature, shrinkage is recorded and foam properties can then be determined by various tests.
[0121] The preparation of molded foam typically involves premixing the polyol component with additives. The isocyanate component is then added to the premix in an amount sufficient to achieve the desired isocyanate index. The reaction mixture is then dispensed by hand or machine into metal molds, which are typically preheated to a temperature of 62°C to 66°C. The reaction mixture was foamed to fill the mold, and after 4 to 5 minutes, the foam was removed from the mold and crushed (physically) to ensure that all...
Embodiment
[0124] The following components were used in the examples.
[0125] Polyol 1 : Propylene oxide adduct of sorbitol containing 12% ethylene oxide as capped and hydroxyl number 33.
[0126] PPD N-phenyl-p-phenylenediamine obtained from SigmaAldrich
[0127] MAA Methyl acetoacetate, available from SigmaAldrich
[0128] TFAA: Trifluoroacetic acid, available from SigmaAldrich
[0129] product preparation :
[0130] Polyol 1 (6000 g, 0.94 moles) and MAA (70 g, 0.60 moles) were added to a 12 L flask under a small nitrogen sparge. The mixture was heated at 140°C for 3 hours until methanol evolution ceased. The mixture was then vacuum stripped to remove any residual MAA and methanol to give a light colored liquid (referred to as polyol 2). Polyol 2 (2700 g), PPD (49.7 g) and 800 mL of toluene were added to a 5 L flask with stirring and a small nitrogen sparge. TFAA (1.0 g) was added and the mixture was heated at reflux (ca. 120° C.) until the theoretical amount of water ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap