A-FABP protein inhibitor and application thereof
A technology of A-FABP and protein inhibitors, applied in the field of A-FABP protein inhibitors, can solve the problems of time-consuming and high cost, and achieve the effect of shortening the cycle and saving R&D investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Screen out A-FABP inhibitors from the FDA drug library
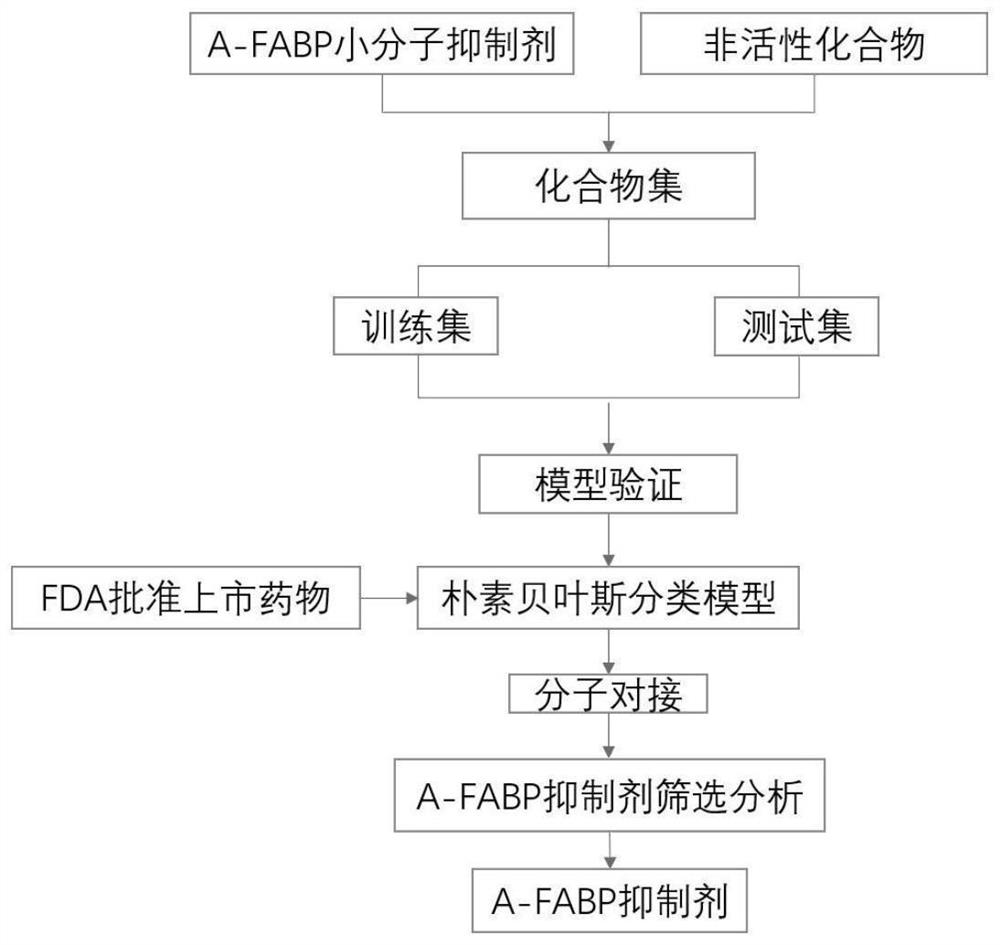
[0029] Aiming at the target of A-FABP, a ligand-based naive Bayesian classification model and a structure-based molecular docking method were used to construct a virtual screening model for A-FABP ( figure 1 ).
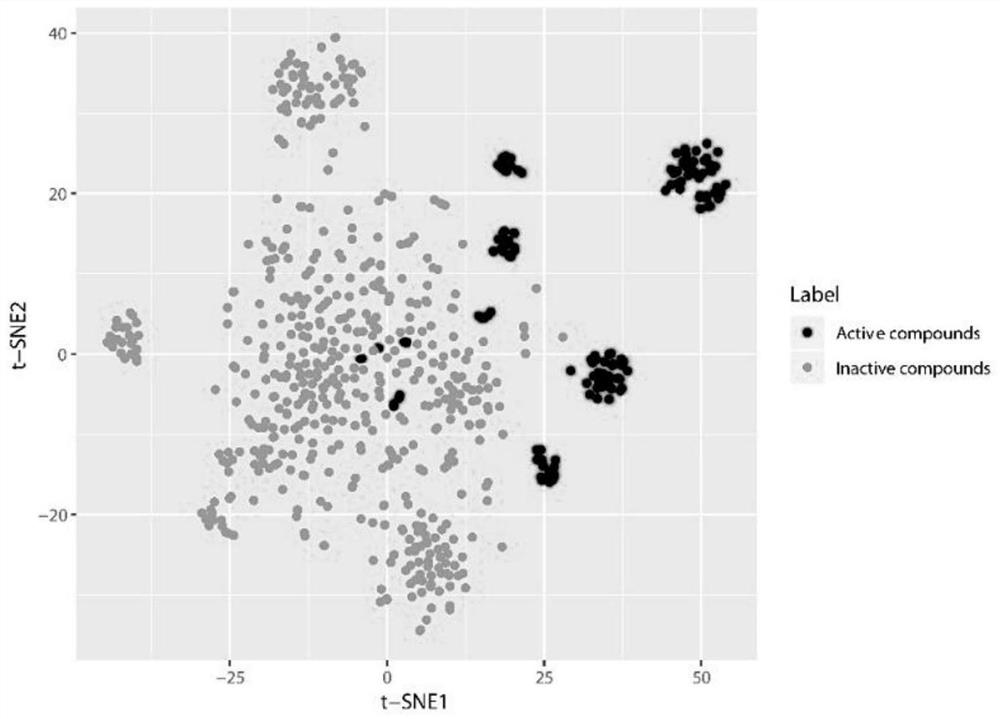
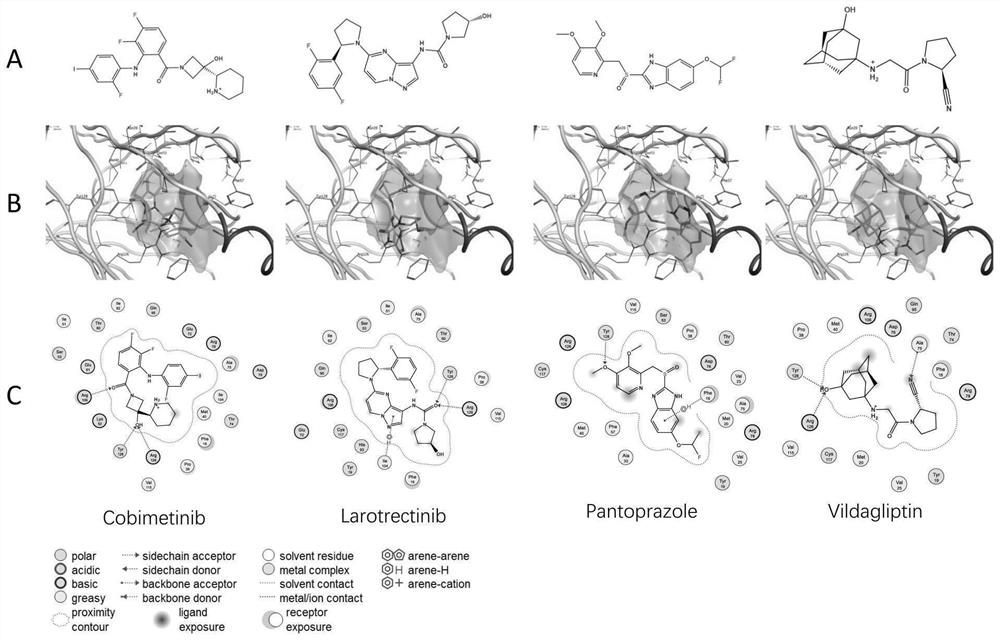
[0030] In the DrugBank database (https: / / go.drugbank.com), the FDA-approved marketed drugs were collected, with a total of 2595 compounds for further research. A-FABP small molecule inhibitors were collected from the ChEMBL database and existing literature to define active compounds. Compounds collected from the ChEMBL database did not overlap with those in the FDA dataset. The corresponding decoys (defined as inactive) were automatically generated from the DUD-E online database (http: / / dude.docking.org / ). As shown in Table 1, the active compound data set and the inactive compound data set were randomly assigned to the training set and the test set at a ratio of 1:4. In the present inventio...
Embodiment 2
[0042] Example 2: Cell-Based Determination of A-FABP Inhibitory Activity of Candidate Compounds
[0043] The A-FABP protein of mouse and human is all 132 amino acids, and homology is 91.7%, and several key amino acid positions (Arg106, Arg126, Tyrl128) that its ligand binds in the amino acid sequence are the same, therefore, candidate compound It can be verified by inhibiting the mouse A-FABP protein.
[0044] RAW 264.7 cells (mouse mononuclear macrophage cell line) were cultured in high-glucose DMEM with 10% fetal bovine serum (FBS, Gibco, USA). The cells were divided into three groups: (1) blank control group; (2) A-FABP protein (1 μg / ml) treatment group; (3) A-FABP protein (1 μg / ml) + candidate compound treatment group. Candidate compounds were diluted to two concentrations (10 μM, 100 μM). In a 37°C, 5% CO2 incubator, the candidate compound was first incubated with the A-FABP protein for 30 minutes, and then added to the cells for 30 minutes.
[0045] Take 38g of Brewer...
Embodiment 3
[0049] Embodiment 3: the detection of candidate compound to cytotoxicity
[0050] The Cell Counting Kit-8 kit (Bioss, BA00208) was used to detect cytotoxicity, and the RAW264.6 cell culture was cultured according to the above requirements, and the experimental groups were set up: (1) blank control group (medium + CCK8 reagent, no cells) ( 2) Reagent control group (cell + medium + CCK8 reagent) (3) Solvent control group (DMSO 100 μM) (cell + medium + DMSO + CCK8 reagent) (4) A-FABP protein (1 μg / ml) treatment group ( Cell + medium + A-FBP4 protein + CCK8 reagent) (5) A-FABP protein (1 μg / ml) + candidate compound treatment group (cell + medium + A-FBP4 protein + candidate compound + CCK8 reagent), the candidate Compounds were diluted to two concentrations (10 μM, 100 μM). In a 37°C, 5% CO2 incubator, the candidate compound was first incubated with the A-FABP protein for 30 minutes, and then added to the cells for 30 minutes. Then add CCK8 reagent and treat at 37° C., 5% CO 2 f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com