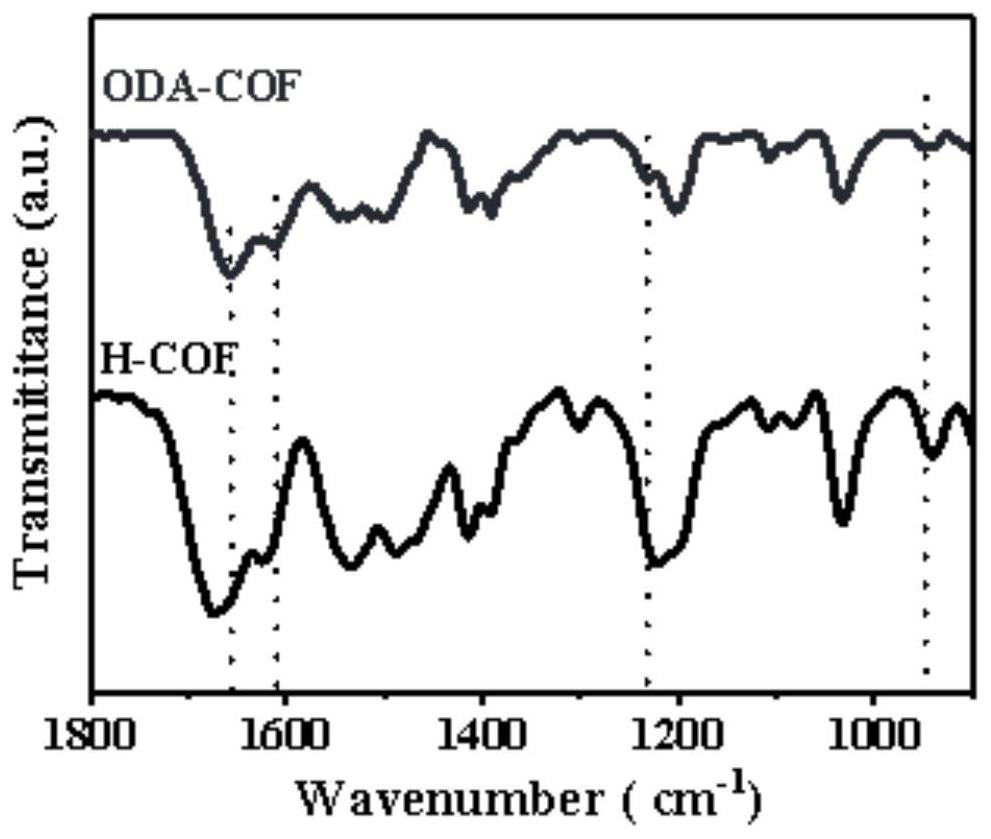
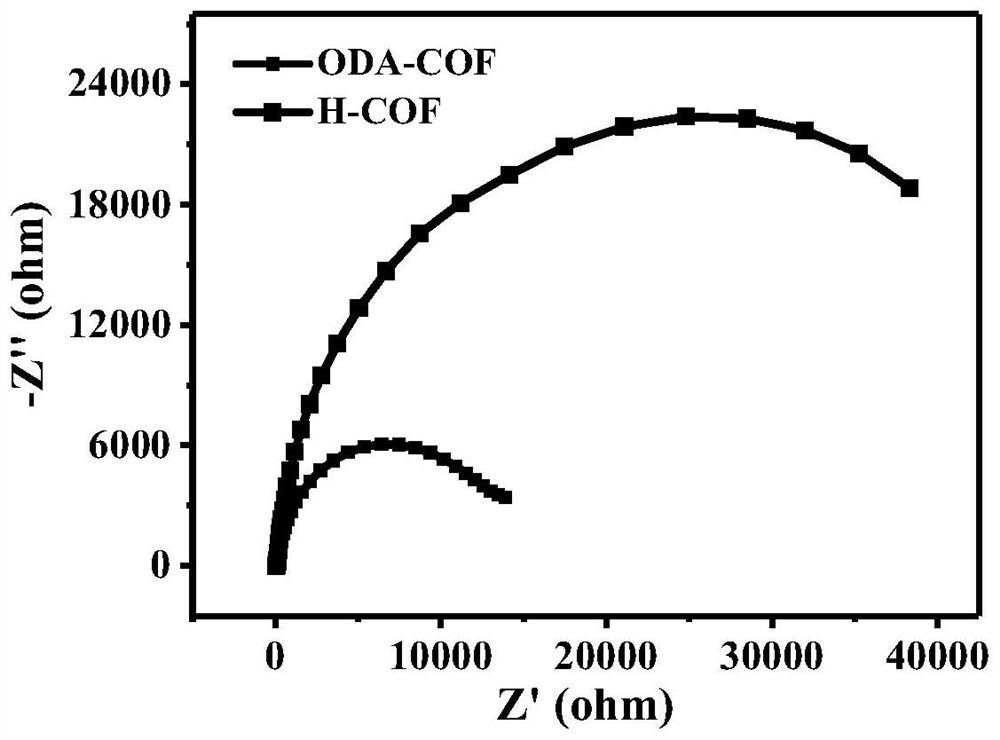
Covalent organic framework material containing oxadiazole connecting element
A technology of covalent organic frameworks and linking groups, applied in organic compound/hydride/coordination complex catalysts, inorganic chemistry, hydrogen production, etc., to achieve the effects of promoting separation and transportation, improving performance, and broadening the absorption range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
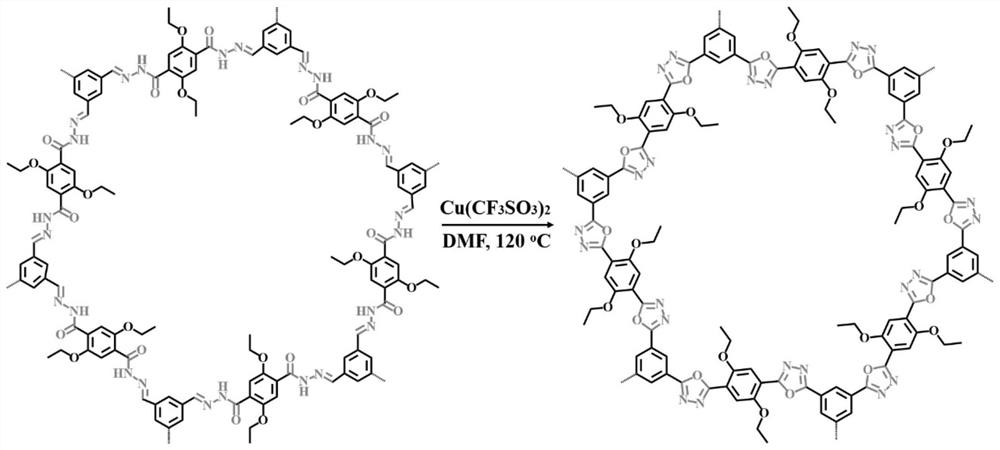
[0031] In a Schlenk tube, add 1,3,5-triformylbenzene (32.4 mg, 0.2 mmol), 2,5-diethoxyterephthalic hydrazide (84.9 mg, 0.3 mmol), mesitylene (6 mL ), anhydrous dioxane (2mL) and aqueous acetic acid solution (0.8mL, 6M), the resulting mixture was sonicated for 10 minutes, and then the air in the tube was removed by three cycles of "freezing-vacuum-thawing" with liquid nitrogen, and the The Schlenk tube was sealed, and after heating at 120°C for 3 days, the resulting solid powder was filtered, washed with tetrahydrofuran and acetone several times, and dried in vacuum at 60°C for 12 hours to obtain COFs containing N-acylhydrazone linkers.
[0032] COFs containing N-acylhydrazone linkers (10 mg, 0.05 mmol), copper triflate (18 mg, 0.05 mmol), cesium carbonate (32 mg, 0.1 mmol) and N,N-dimethylformamide ( 15mL) into a dry flask and heated at 120°C for 72 hours. During the reaction, the same amount of copper trifluoromethanesulfonate (that is, both 18mg, 0.05mmol) was added twice (a...
Embodiment 2
[0038] In a Schlenk tube, add 2,4,6-tris(4-formylphenyl)-1,3,5-triazine (80.5 mg, 0.2 mmol), 2,5-diethoxyterephthalamide Hydrazide (84.6mg, 0.3mmol), mesitylene (2.7mL), anhydrous dioxane (1.3mL) and aqueous acetic acid (0.4mL, 6M), the resulting mixture was sonicated for 10 minutes, and then washed with liquid nitrogen "Freeze-vacuum-thaw" cycled three times to remove the air in the tube, sealed the Schlenk tube, heated at 120 °C for 3 days, filtered the resulting solid powder, washed with tetrahydrofuran and acetone several times, and dried in vacuum at 60 °C COFs containing N-acylhydrazone linkers were obtained after 12 hours.
[0039] COFs containing N-acylhydrazone linkers (13 mg, 0.05 mmol), copper triflate (18 mg, 0.05 mmol), cesium carbonate (32 mg, 0.1 mmol) and ultra-dry N,N-dimethylmethane Amide (15mL) was packed into a dry flask and heated at 115°C for 72 hours. During the reaction, the same amount of copper trifluoromethanesulfonate (that is, 18mg, 0.05mmol) was ...
Embodiment 3
[0041] In a Schlenk tube, add 1,3,5-tris(4-formylphenyl)benzene (78.0 mg, 0.2 mmol), trimellitic hydrazide (50.4 mg, 0.2 mmol), mesitylene (20 mL), Anhydrous dioxane (20 mL) and acetic acid aqueous solution (4 mL, 6M), the resulting mixture was sonicated for 10 minutes, and then the air in the tube was removed by "freeze-vacuum-thaw" cycle with liquid nitrogen three times, and the Schlenk tube was sealed , after heating at 120 °C for 3 days, the resulting solid powder was filtered, washed with THF and acetone several times, and dried under vacuum at 60 °C for 12 h to obtain COFs containing N-acylhydrazone linkers.
[0042] COFs containing N-acylhydrazone linkers (9.8 mg, 0.05 mmol), copper triflate (18 mg, 0.05 mmol), potassium carbonate (13.8 mg, 0.1 mmol) and ultra-dry toluene (15 mL) were charged The dried flask was heated at 120°C for 72 hours. During the reaction, the same amount of copper trifluoromethanesulfonate (ie, both 18 mg, 0.05 mmol) was added twice (at an interv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com