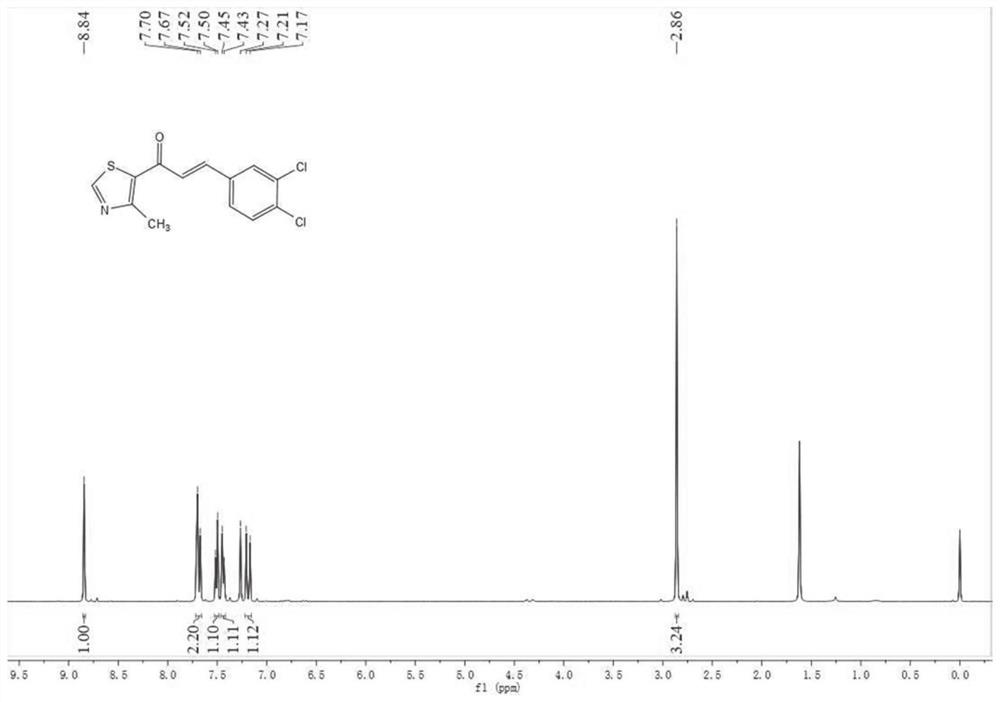
Application of compound as CYP2E1 inhibitor
A CYP2E1, Compound Technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0199] Example 1 Preparation of human liver microsomes
[0200] Using differential centrifugation, the liver samples were taken out, thawed, and weighed. Add 50mM Tris-HCl (pH=7.0) (containing 150mM KCl, 2mM EDTA) buffer at a ratio of 1:4 (W / V), grind with a glass homogenizer to make liver homogenate. Centrifuge at 9000×g at 4°C for 20 minutes, take the supernatant and centrifuge at 100,000×g at 4°C for 60 minutes; resuspend the obtained pellet in 4 mL of 0.15M Tris-HCl (pH=7.6), and centrifuge at 100,000 g at 4°C for 60 minutes; take the precipitate Add 0.25M sucrose resuspension at a ratio of 1:2 (W / V), and finally prepare 2 mL of microsomal suspension per gram of liver tissue, store in liquid nitrogen overnight after aliquoting, and transfer to -80°C for a long-term Save for later. All the above operations were carried out in an ice bath. Microsomal protein content (mg / mL) was determined by Bradford method.
[0201] This method is used for liver microsomes of patients w...
Embodiment 2
[0202] The synthesis of embodiment 2 SMIO
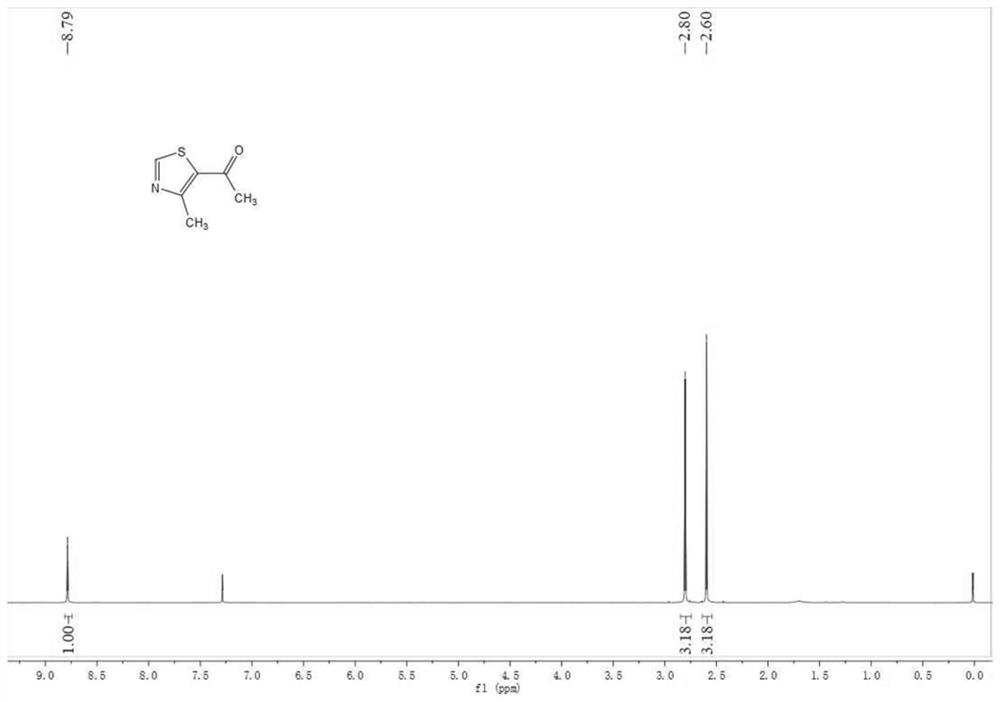
[0203] Ethyl 4-methylthiazole-5-carboxylate (1mol) and NaOH (1.6mol), the solvent is a mixed solution of ethanol and water, react overnight at room temperature, TLC detection (pure ethyl acetate), after the reaction is complete, ethanol Evaporate to dryness under reduced pressure, adjust the pH to 2-3 with concentrated sulfuric acid, obtain a solid after suction filtration, wash and dry. 4-methylthiazole-5-carboxylic acid (1mol) and DDC (1mol) were stirred in anhydrous tetrahydrofuran at room temperature. After activation for 2-3h, dimethylhydroxylamine hydrochloride (1.2mol) was added, and triethylamine (1.5 mol) stirred at room temperature overnight, and detected by TLC (PE:EA=3:1). After the reaction was complete, tetrahydrofuran was evaporated to dryness under reduced pressure, extracted three times with ethyl acetate, both sides were washed with saturated aqueous sodium bicarbonate solution, dried over anhydrous magnesium sulfa...
Embodiment 3
[0205] The synthesis of embodiment 3 compound SMI7
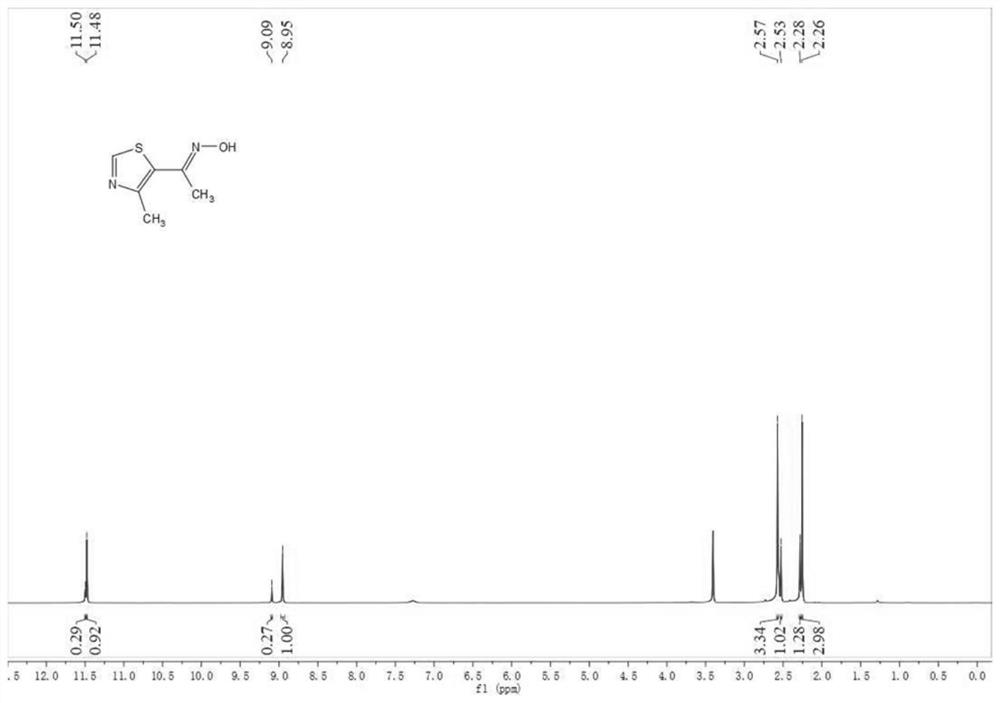
[0206] Take hydroxylamine hydrochloride (3mmol) in a round-bottomed flask, add 3mL of ethanol, stir at room temperature 25°C for 10min, add 3mL of 1M NaOH solution, then add SMI0 (3mmol), reflux in an oil bath at 80°C, after TLC monitors that the reaction is complete, add 10% dilute hydrochloric acid Neutralize the reaction solution, extract with water and ethyl acetate, combine the organic phases, dry over anhydrous magnesium sulfate, remove the magnesium sulfate by suction filtration, concentrate the filtrate under vacuum, separate by silica gel column chromatography, the eluent and its ratio are petroleum ether: Ethyl acetate=1:2, obtain compound SMI7, the nuclear magnetic detection data of product are as follows (as figure 2 shown): 1 H NMR(400MHz,DMSO)δ11.50(s,2 / 3H),11.48(s,1 / 3H),9.09(s,1 / 3H),8.95(s,2 / 3H),2.57(s,2 / 3H),2.53(s,1 / 3H),2.28(s,1 / 3H),2.26(s,2 / 3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com