Method for constructing ionic gel drug-loaded coating on surface of biodegradable metal
A technology of ion gel and metal surface, which is applied in the fields of coating, medical science, surgery, etc., can solve the problem that it is difficult to take into account the corrosion of zinc-based metals to control the biological functions of zinc-based metals, the effect is not very ideal, and the surface modification method is single, etc. problems, achieve the effects of reducing the release of zinc ions, promoting the adhesion of osteoblasts, and improving the anti-inflammatory properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] This example provides a method for constructing an ion gel drug-loaded coating on a biodegradable metal surface, including the following steps:
[0043] a. Pretreatment of zinc flakes: Grind the zinc flakes gradually with 600#, 1200#, 2000# sandpaper, then clean them sequentially with deionized water and absolute ethanol under ultrasonic conditions, and finally dry them for later use;
[0044] b. Phosphorylation treatment of zinc flakes: place the polished zinc metal flakes in the above steps in 7.68g / L NaH 2 PO 4 ,1.85g / L Zn(NO 3 )·6H 2 O mixed solution, soaked at 50°C for two hours, then cleaned with deionized book, dried for later use;
[0045] c. Configuration of gel solution and ionic solution: Mix 10g / L carboxymethyl chitosan solution and 10g / L gelatin solution at 50°C to form carboxymethyl chitosan / gelatin mixed solution ; The Zn(NO of 5.94g / L 3 )·6H 2 O and 0.05g / L aspirin are mixed under the condition of 50°C to configure a zinc ion / carrier drug mixed sol...
experiment example 1
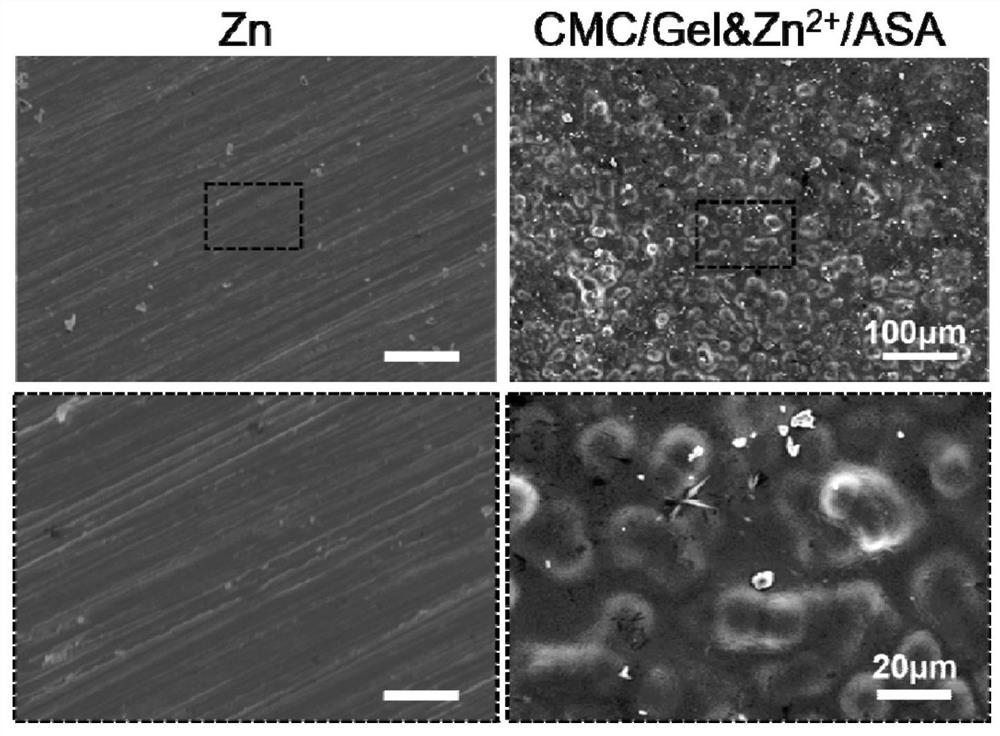
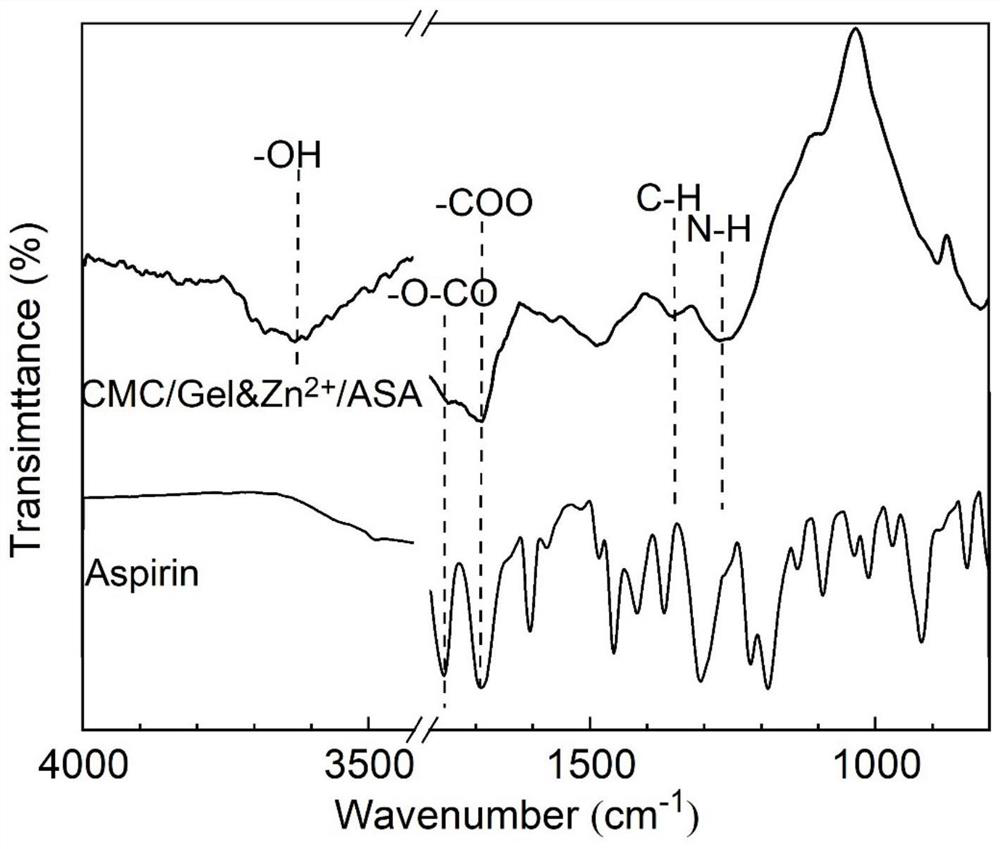
[0051] In this example, the sample of the ion gel drug-loaded coating prepared in Example 1 was tested by infrared spectroscopy by reflection method.
[0052] Infrared spectroscopy test results such as figure 2 shown. at 1260cm -1 At , the characteristic peak of N-H appeared in the coating, which was derived from gelatin, indicating that gelatin was successfully built in the coating. at 1346cm -1 At , the characteristic peak of C-H appeared in the coating, which was derived from carboxymethyl chitosan, indicating that carboxymethyl chitosan was successfully built in the coating. at 1747cm -1 , the characteristic peak of -O-CO appeared in both the coating and the aspirin powder, indicating that the aspirin drug was loaded in the coating.
experiment example 2
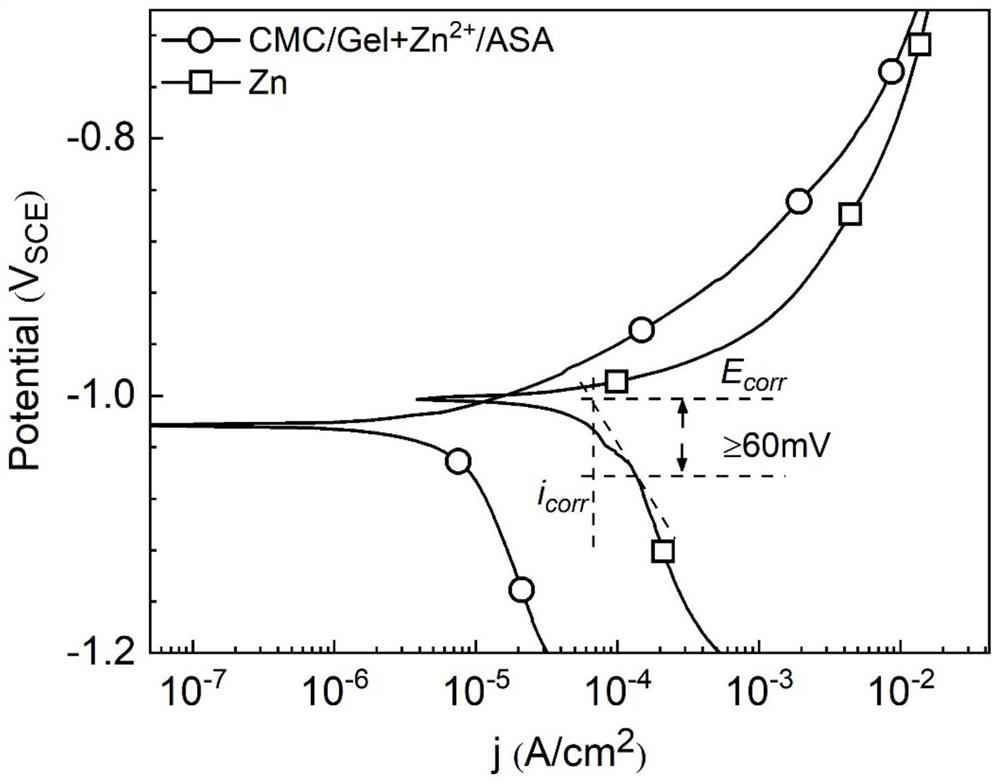
[0054] In this example, a conductive silver glue is coated on the surface of the polished zinc sheet and the coated zinc sheet prepared in Example 1 to connect it with the wire, and then seal the other parts of the sample except the coated surface with silicon rubber. Finally, put the processed sample into the three-way tube with Hank's solution as the electrolyte, in which the sample is used as the working electrode, the metal platinum is used as the counter electrode, and the calomel electrode is used as the reference electrode. Using IM6 electrochemical The workstation is used to test the potentiodynamic polarization curve of the sample.
[0055] The polarization curve of the sample in this example is as follows image 3 shown. As shown in the figure, the self-corrosion potential E of the sample after coating modification corr Correction, self-corrosion current i corr decreased, indicating that the coating can slow down the corrosion of the zinc substrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com