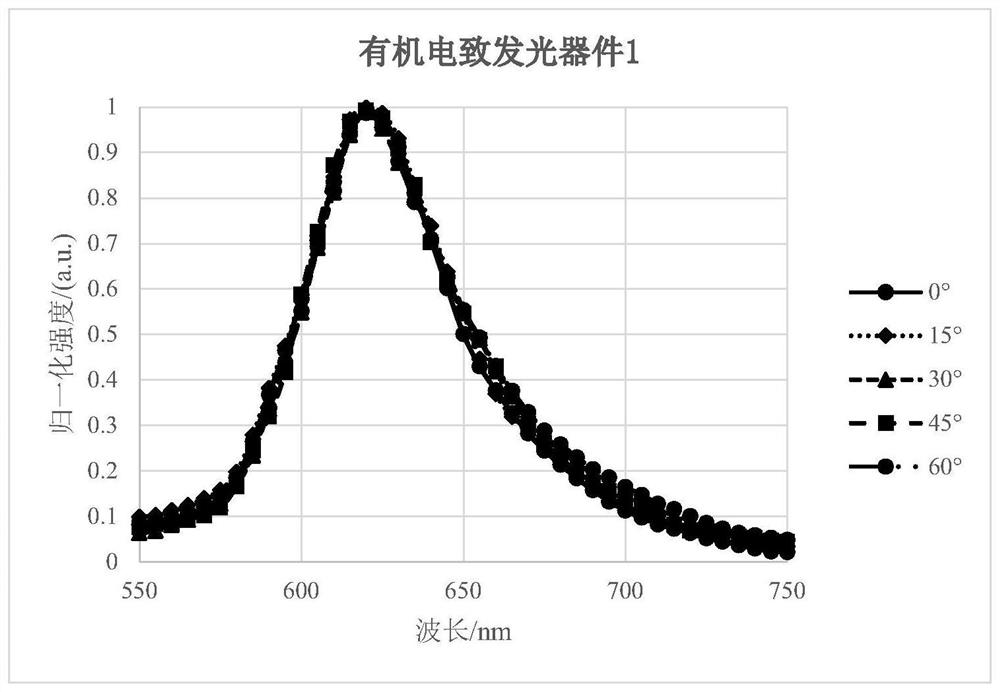
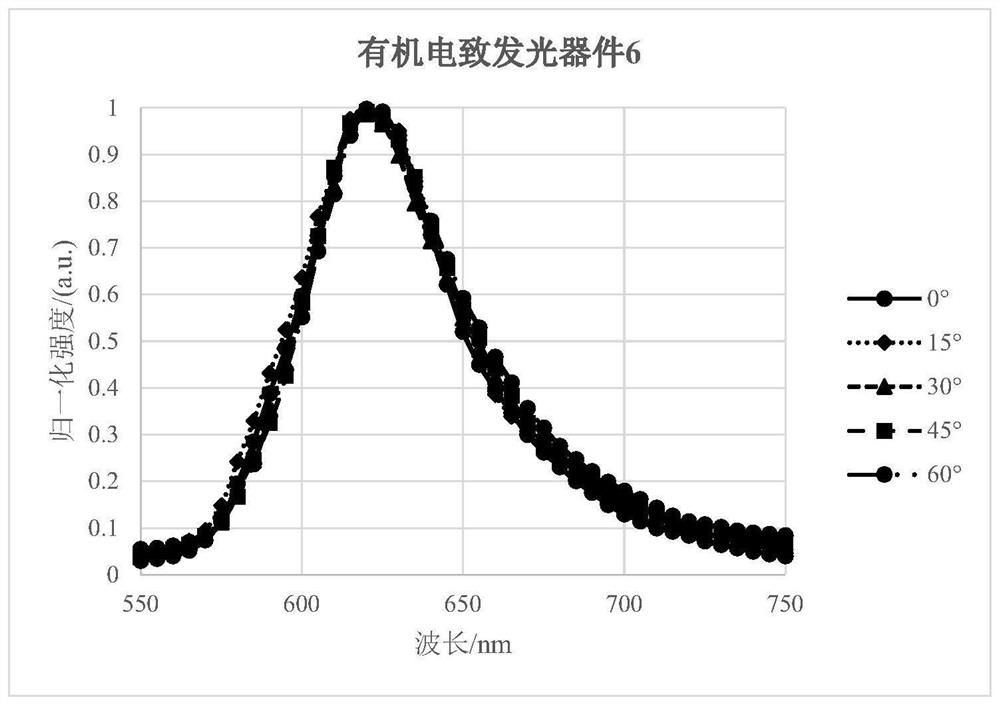
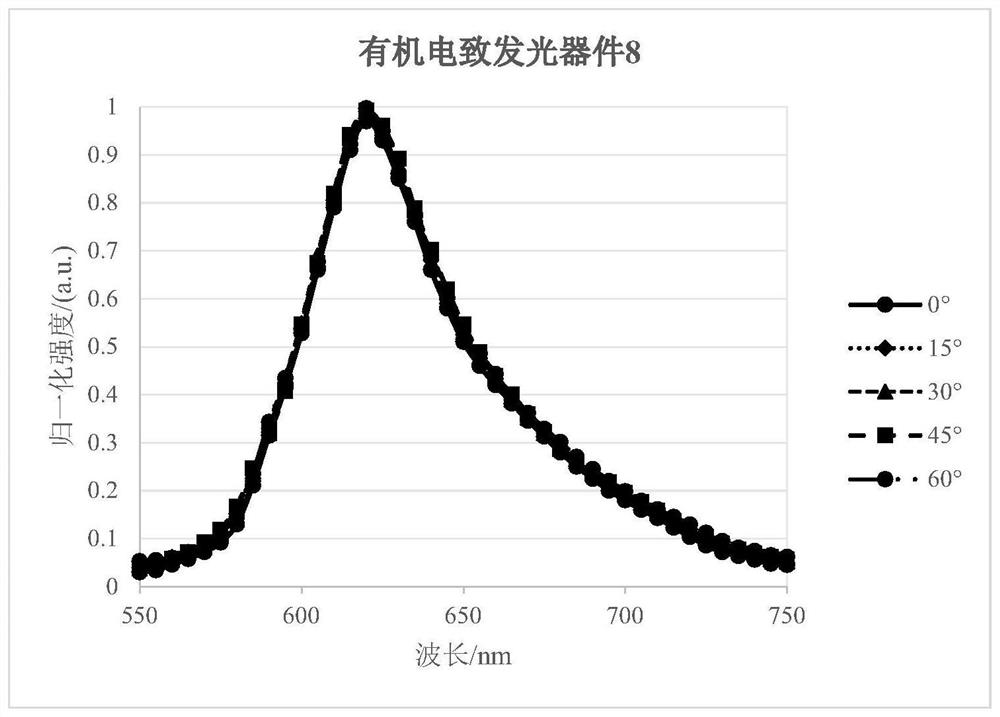
Organic light-emitting device comprising light extraction layer
A technology of electroluminescent devices and light extraction layers, which is applied in the direction of electric solid devices, electrical components, semiconductor devices, etc., can solve the problems of restricting the development of top-emitting OLEDs, affecting the color purity of devices, and increasing the angle dependence, so as to avoid the problems of devices The effect of sharp roll-off of efficiency, improvement of light extraction efficiency, and improvement of light extraction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] [Synthesis Example 1] Preparation of a-2
[0120]
[0121] d-1 (25.80 g, 150 mmol), e-1 (33.75 g, 152 mmol), K 2 CO 3 (34.55g, 250mmol), 500mL mixed solvent (toluene:ethanol:water=2:1:1), and then replaced the air with nitrogen three times, and then added Pd (PPh 3 ) 4 (1.16g, 1.0mmol), under the protection of nitrogen, the reaction was stirred at reflux temperature for 3h, after the reaction was completed, cooled to room temperature, liquid separation, after the organic phase was separated from the water phase, the organic phase was washed with water, washed with anhydrous sulfuric acid Dry over sodium, filter to remove anhydrous sodium sulfate, concentrate under reduced pressure and rotate to evaporate the solvent, then recrystallize the crude product with toluene to obtain a-2 (27.88 g, yield 69%); HPLC purity ≥99.32%.
[0122] According to the above-mentioned synthetic mode, the present invention also synthesized the relevant intermediates in the following tab...
Embodiment 2
[0124] [Synthesis Example 2] Preparation of Compound 1
[0125]
[0126] Preparation of Intermediate A-1:
[0127] A-1 (18.20g, 83mmol), b-1 (22.65g, 80mmol), sodium tert-butoxide (11.53g, 120mmol), 300mL toluene solvent were added to the reaction flask in turn, and then the air was replaced by nitrogen three times, and then Join Pd 2 (dba) 3 (0.73g, 0.8mmol), tri-tert-butylphosphine (0.32g, 1.6mmol), under the protection of nitrogen, the reaction was stirred at reflux temperature for 4.5h, after the reaction was completed, cooled to room temperature, the reactant was filtered through diatomaceous earth After filtration, the solvent was removed by rotary distillation under reduced pressure, and the obtained crude product was recrystallized from ethyl acetate to obtain Intermediate A-1 (28.66 g, yield 85%); HPLC purity≥99.57%.
[0128] Preparation of compound 1:
[0129] Add intermediate A-1 (26.98g, 64mmol), c-1 (17.83g, 60mmol), sodium tert-butoxide (8.64g, 90mmol), 20...
Embodiment 3
[0131] [Synthesis Example 3] Preparation of Compound 38
[0132]
[0133] Replace a-1, b-1, c-1 in Synthesis Example 2 with equimolar a-2, b-2, c-2, and follow the same preparation method as Synthesis Example 2 to obtain compound 38 ( 36.30 g); HPLC purity > 99.74%.
[0134] Mass spectrum m / z: 737.2743 (theoretical: 737.2719). Theoretical element content (%) C 56 H 35 NO: C, 91.15; H, 4.78; N, 1.90. Measured element content (%): C, 91.18; H, 4.82; N, 1.88.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com