Bionic cell-containing large bone cartilage biological scaffold and preparation method thereof
A bio-scaffold, osteochondral technology, applied in the field of biomedical tissue engineering, can solve the problems of surrounding tissue fit, single, unable to meet the functional requirements of natural osteochondral cartilage surface structure requirements, etc., to speed up repair and promote integrated repair and reconstruction. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
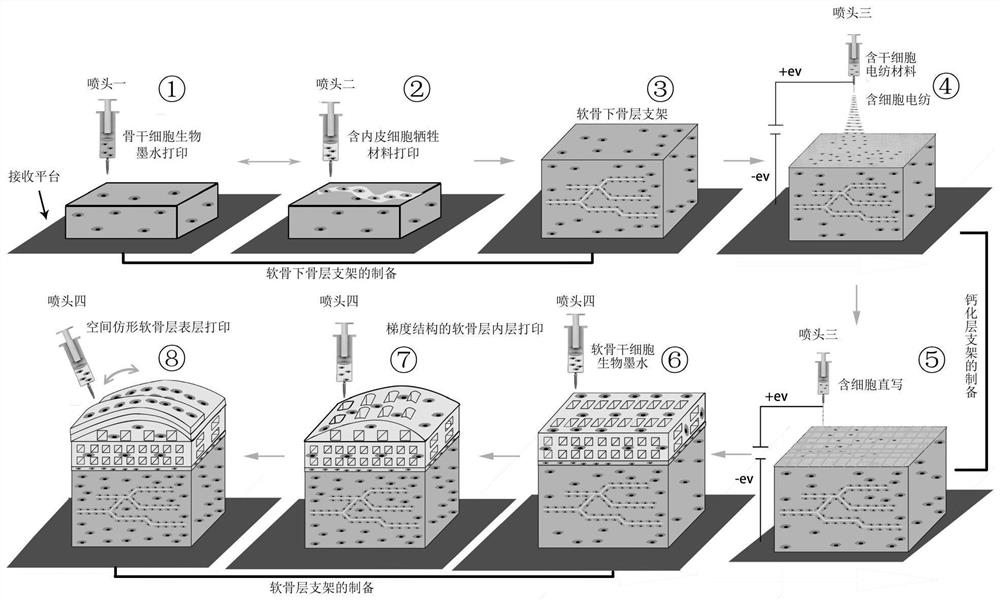
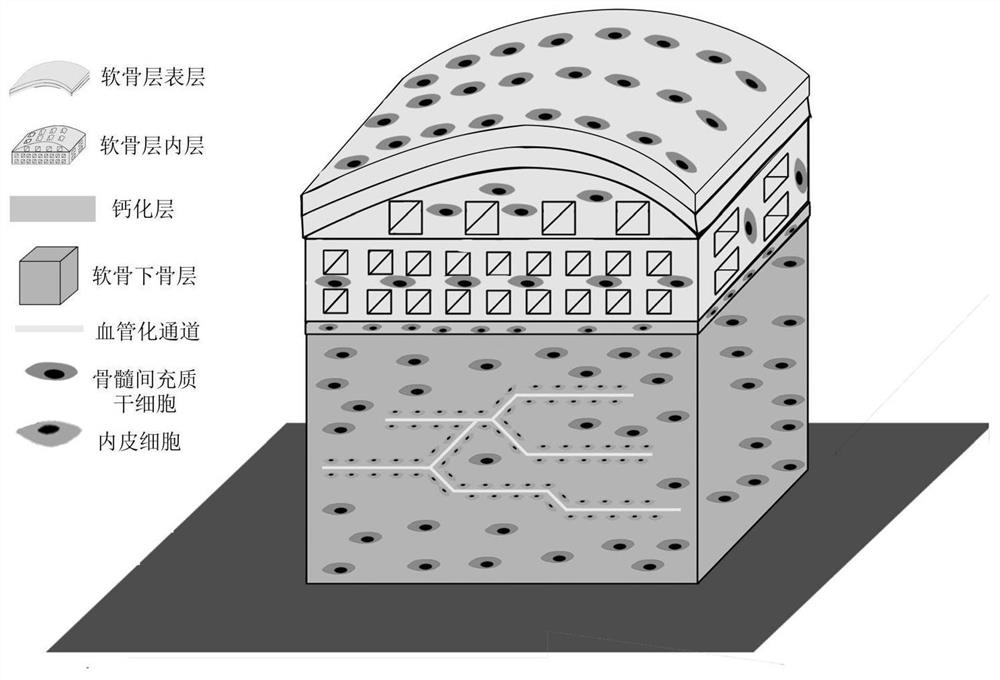
[0031] The invention provides a method for preparing a bionic cell-containing large bone and cartilage bioscaffold, comprising the following steps:
[0032] The subchondral bone material containing bone marrow mesenchymal stem cells is subjected to the first extrusion 3D printing, and the sacrificial material containing human umbilical vein endothelial cells is used in the formed subchondral bone scaffold structure for the second extrusion 3D printing, alternately Performing the first extrusion 3D printing and the second extrusion 3D printing to obtain a subchondral bone layer containing a prefabricated vascularized network structure;
[0033] Using electrospun materials containing bone marrow mesenchymal stem cells, electrospinning is performed on the subchondral bone layer, and a cell-containing nanofiber isolation membrane is formed on the upper surface of the subchondral bone layer;
[0034] Continue to use the electrospun material containing bone marrow mesenchymal stem c...
Embodiment 1
[0077] according to figure 1 Shown flow process carries out the scheme of embodiment 1:
[0078] a. Preparation of bone stem cell bioink: using PBS buffer as solvent, gelatin (10%), sodium alginate (5%), bone marrow mesenchymal stem cells (5×10 6 cells / g) were mixed and prepared, and 5% of BMP-2 protein was added (w / w, relative to the total mass of bone stem cell bioink); 10 mL of the prepared bone stem cell bioink was added to a medical syringe, and the syringe Installed on print head one;
[0079] Preparation of cell-containing sacrificial material: using PBS buffer as solvent, collagen (5%, w / v), PVA (10%, w / v) and human umbilical vein endothelial cells (5×10 6 cells / g) were mixed and prepared; then 10 mL of the prepared cell-containing sacrificial material was added into a medical syringe, and installed on the printing nozzle 2;
[0080] b. Preparation of electrospun materials containing stem cells: PBS buffer as solvent, PEO (15%), sodium alginate (5%) and human umbili...
Embodiment 2
[0088] The difference between this embodiment and embodiment 1 is only:
[0089] Bone stem cell bioink, the solvent is HBSS solution (Hank's balanced salt solution), using methacrylated gelatin (12% w / v), hydroxyapatite (5% w / w) and bone marrow mesenchymal stem cells (5 ×10 6 cells / g) were mixed and prepared, and 0.2% (w / w, relative to the total mass of the stem cell bioink) was added with LAP photoinitiator;
[0090] The solvent of chondrocyte stem cell bioink is HBSS solution (Hank's balanced salt solution), using methacrylated gelatin (10% w / v), hyaluronic acid (10% w / v) and bone marrow mesenchymal stem cells (5×10 6 cells / g) were mixed and prepared, and 0.2% (w / w, relative to the total mass of cartilage stem cell bioink) was added with LAP photoinitiator;
[0091] Place the prepared bulk osteochondral scaffold under blue light with a wavelength of 405nm for cross-linking;
[0092] Other steps are with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com