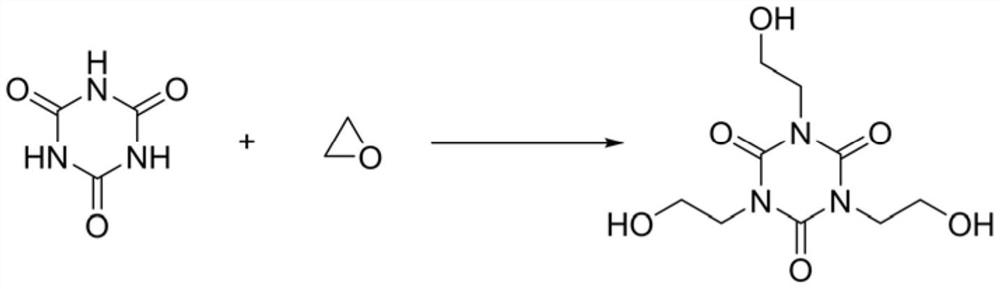
Preparation method of tris (2-hydroxyethyl) isocyanurate
A technology of isocyanurate and hydroxyethyl, applied in the field of preparation of triisocyanurate, can solve the problems of affecting product purity, unstable yield, narrow operating window, etc., and achieve reaction yield and crude product purity Simplify, improve product yield and purity, and reduce the effects of polymerization side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The present embodiment provides a kind of preparation method of three (2-hydroxyethyl) isocyanurate, comprises the steps:
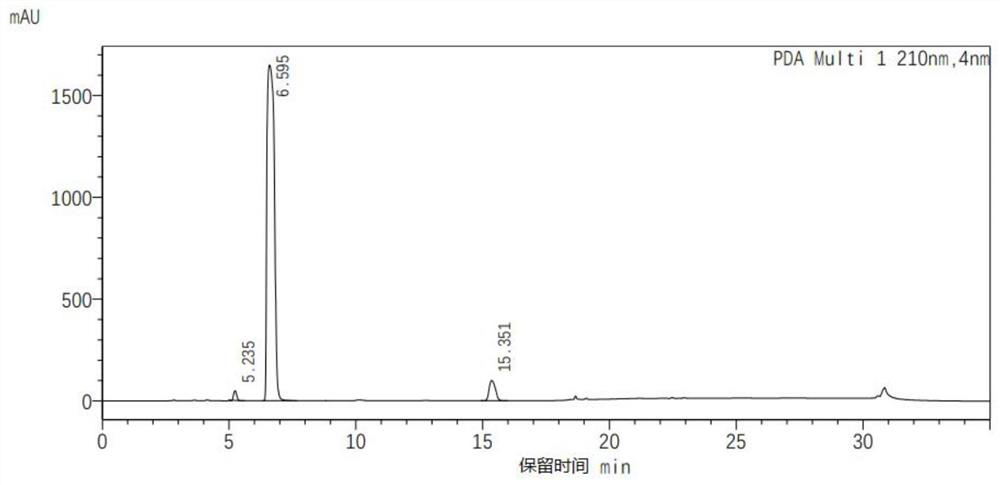
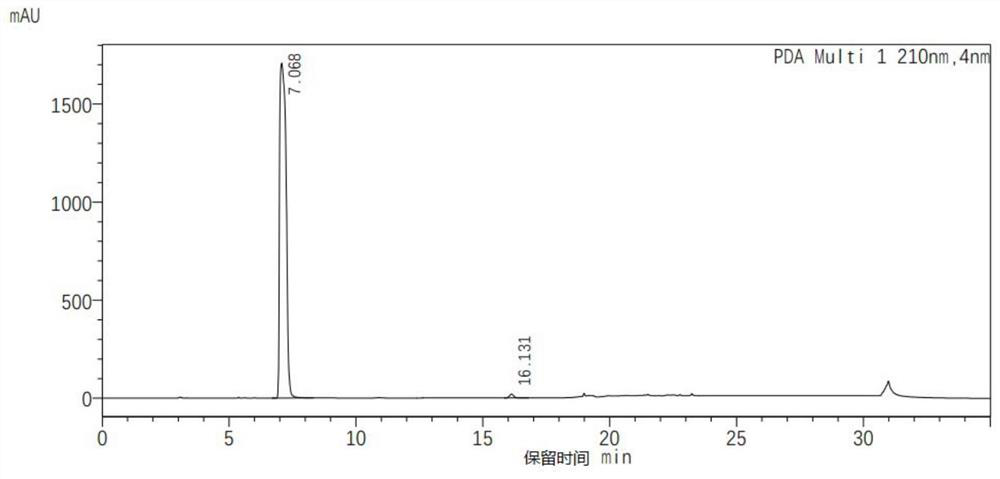
[0027] 412gN,N-dimethylacetamide, 100g cyanuric acid (purity 98%), 0.5g dimethylamine and 1.2g BF 3 ·Et 2 O(BF 3 content is 46.5wt%) into a 3L reactor, heated up to 110°C, then pumped 108g of ethylene oxide into the reaction by the feeding pump, controlled the reaction temperature to 110°C, and the reaction time was 2h. After the reaction, the reaction liquid Cool down to 30°C, remove N,N-dimethylacetamide by distillation under reduced pressure to obtain 257 g of a wet crude product of tris(2-hydroxyethyl)isocyanurate; then add 500 mL of acetone to the wet crude product and stir at room temperature Filter and dry after 0.5 hour to get three (2-hydroxyethyl) isocyanurate 186g, yield 92%, this three (2-hydroxyethyl) isocyanurate product is analyzed its purity through high performance liquid chromatography 94%.
Embodiment 2
[0029] The present embodiment provides a kind of preparation method of three (2-hydroxyethyl) isocyanurate, comprises the steps:
[0030] Add 380g of pyridine and 100g of cyanuric acid (purity 98%) into a 3L reactor, raise the temperature to 110°C, then pump 115g of ethylene oxide into the reaction by the feeding pump, control the reaction temperature to 110°C, and the reaction time to 2.5h , after the reaction, the reaction solution was cooled to 35° C., and pyridine was removed by distillation under reduced pressure to obtain 264 g of a wet crude product of tris (2-hydroxyethyl) isocyanurate. Then, 500 mL of acetone was added to the wet crude product, and stirred at room temperature for 0.5 After hours, filter and dry to get three (2-hydroxyethyl) isocyanurate 192g, yield 95%, and this three (2-hydroxyethyl) isocyanurate product is analyzed by high performance liquid chromatography and its purity is 90%.
Embodiment 3
[0032] The present embodiment provides a kind of preparation method of three (2-hydroxyethyl) isocyanurate, comprises the steps:
[0033] 2027g N-methylpyrrolidone, 1006g cyanuric acid (98% purity), 5g Cs 2 CO 3 and 6g BF 3 ·Et 2 O(BF 3 content is 46.5wt%) into a 20L reactor, heated up to 110°C, then pumped 1091g of ethylene oxide into the reaction by feeding pump, controlled reaction temperature was 110°C, and reaction time was 1.5h. Cool the liquid to 40°C, discharge it into a 50L three-in-one equipment, add 8580g of acetonitrile, stir at 60°C for 0.5 hours, then cool down to 10°C, filter and dry in the three-in-one equipment to obtain tris(2-hydroxyethyl)iso Cyanurate 1927g, yield 95%, this tris (2-hydroxyethyl) isocyanurate product is analyzed by high performance liquid chromatography and its purity is 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com