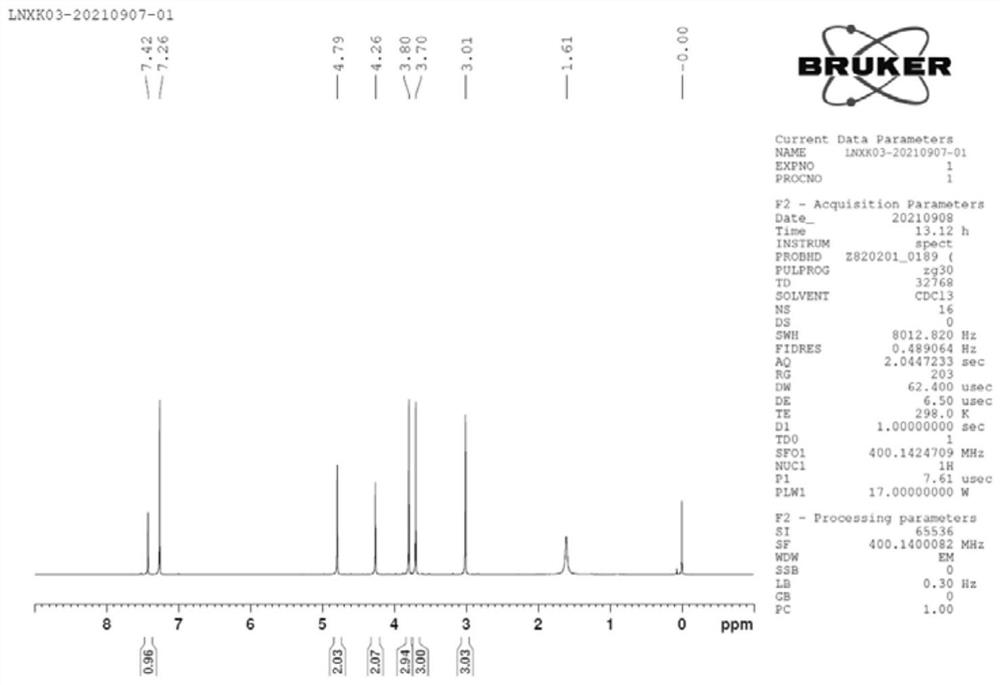
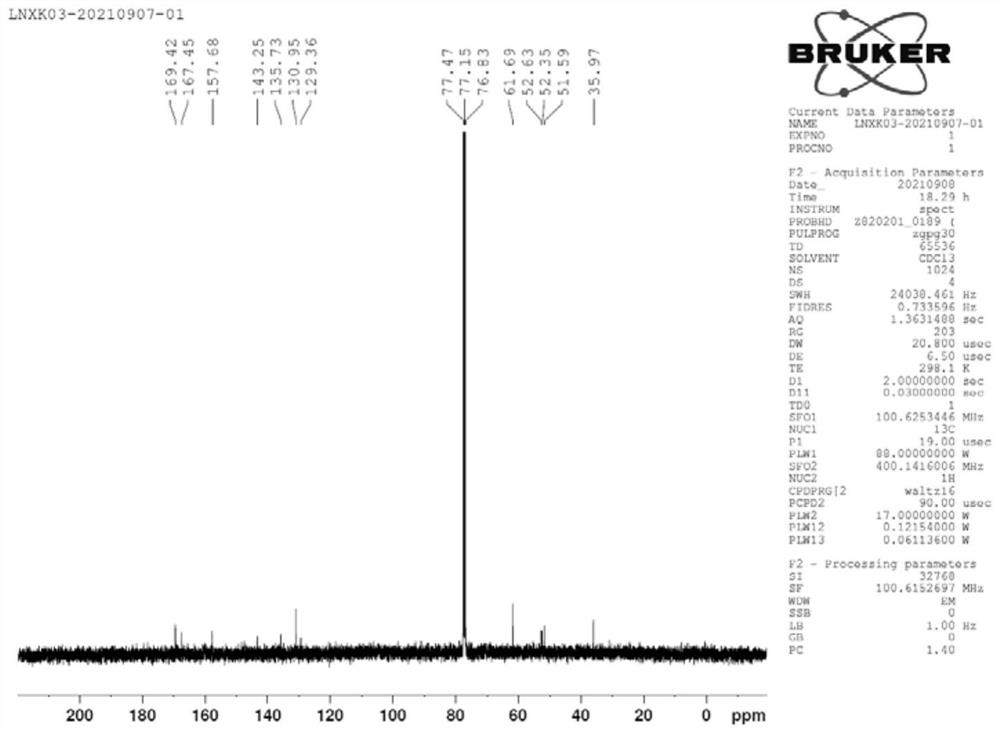
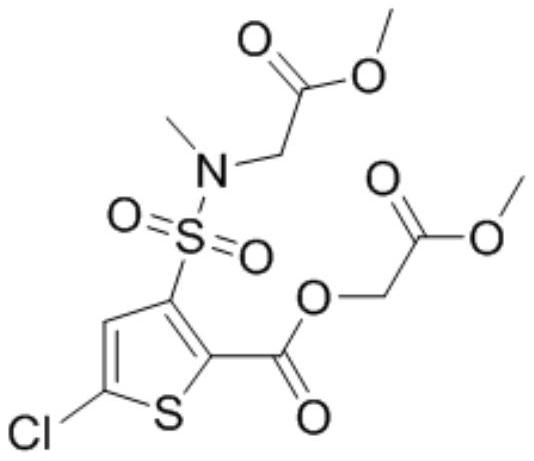
Preparation method of lornoxicam impurity
A technology for lornoxicam and impurities, which is applied in the field of preparation of lornoxicam impurities, can solve the problems of process impurities, side reactions of methyl bromoacetate, etc., and achieve short synthesis routes, high product purity, and simple synthesis methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Under the protection of nitrogen, add 200ml of acetonitrile and 20.00g of 5-chloro-3-methylsulfonamidethiophene-2-carboxylic acid into a 500mL four-necked bottle, cool down to 0-5°C, and add 24.43g of phosphorus pentachloride Reaction 1h, obtains the acetonitrile solution of intermediate 1;
[0044] (2) Control the temperature at 0-5°C, add 8.46 g of methyl glycolate dropwise to the acetonitrile solution of intermediate 1, after the dropwise addition, slowly raise the temperature to 22°C, and keep it warm for 4 hours, TLC detects the reaction, 5-chloro - The reaction of 3-methylsulfonamidethiophene-2-carboxylic acid is complete, the reaction system is cooled to 0-5°C, 200ml of purified water is added dropwise, and after 2 hours of heat preservation at 0-5°C, 25.30g of intermediate 2 wet product is obtained by suction filtration After vacuum drying at 40°C for 6 hours, 21.80 g of intermediate 2 was obtained with a yield of 85% and a purity of 97.2%;
[0045] (3) Und...
Embodiment 2
[0047] (1) Under the protection of nitrogen, add 500ml of acetonitrile and 50.00g of 5-chloro-3-methylsulfonamidethiophene-2-carboxylic acid into a 1000mL four-necked bottle, cool down to 0-5°C, and add 61.03g of phosphorus pentachloride Reaction 0.5h, obtains the acetonitrile solution of intermediate 1;
[0048](2) Control the temperature at 0-5°C, add 21.14 g of methyl glycolate dropwise to the acetonitrile solution of intermediate 1, after the addition is complete, slowly raise the temperature to 24°C, and keep it warm for 2 hours, TLC detects the reaction, 5-chloro - The reaction of 3-methylsulfonamidethiophene-2-carboxylic acid is complete, the reaction system is lowered to 0-5°C, 200ml of purified water is added dropwise, and after 3 hours of heat preservation at 0-5°C, 62.18g of intermediate 2 wet product is obtained by suction filtration , after vacuum drying at 45°C for 5 hours, 55.76g of intermediate 2 was obtained with a yield of 87% and a purity of 97.5%;
[0049]...
Embodiment 3
[0051] (1) Under the protection of nitrogen, add 1000ml of acetonitrile and 100.00g of 5-chloro-3-methylsulfonamidethiophene-2-carboxylic acid into a 2000mL four-necked bottle, cool down to 0-5°C, and add 122.16g of phosphorus pentachloride Reaction 3h, obtains the acetonitrile solution of intermediate 1;
[0052] (2) Control the temperature at 0-5°C, add 42.28 g of methyl glycolate dropwise to the acetonitrile solution of intermediate 1, after the dropwise addition, slowly raise the temperature to 20°C, and keep it warm for 5 hours, TLC detects the reaction, 5-chloro - The reaction of 3-methylsulfonamidethiophene-2-carboxylic acid is complete, the reaction system is cooled to 0-5°C, 1000ml of purified water is added dropwise, and after 1 hour of heat preservation at 0-5°C, 120.55g of intermediate 2 wet product is obtained by suction filtration After vacuum drying at 55°C for 3 hours, 112.30 g of intermediate 2 was obtained, with a yield of 88% and a purity of 96.8%;
[0053]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com