Cobalt-copper bimetallic catalyst for directly preparing low-carbon alcohol from synthesis gas as well as preparation method and use method of cobalt-copper bimetallic catalyst
A bimetallic catalyst, synthesis gas technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, preparation of organic compounds, chemical instruments and methods, etc. Stability, separation and particle agglomeration, etc., to achieve excellent low-carbon alcohol space-time yield, excellent target product selectivity and reaction stability, high dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation method of the above catalyst is as follows: firstly, the layered copper silicate substrate is prepared by distilling ammonia, and then it is subjected to cobalt ion exchange, and the obtained mixed solid sample is roasted in air, pressed into tablets, sieved, and then subjected to a certain temperature. The finished catalyst is obtained after hydrogen reduction.
[0034] Above-mentioned preparation method comprises the following steps:
[0035] ⑴After mixing a certain amount of copper salt, deionized water and ammonia water at room temperature to form a uniform cuproammonia solution, add the silica precursor (copper loading 30-50wt.%) drop by drop, and stir and age for 4 -7h.
[0036] When preparing the base copper-silicon material, the copper loading is preferably 36 wt.%. The copper salt is copper nitrate, copper acetate or copper chloride, preferably copper nitrate. The silica precursor is silica sol, sodium silicate or orthosilicic acid, preferabl...
Embodiment 1
[0044] Catalyst precursor preparation:
[0045] Weigh 20.3g of copper nitrate trihydrate and 54.8mL of ammonia water and dissolve in 100mL of deionized water to form a cuproammonia solution, then add 22.25mL of silica sol dropwise and stir at room temperature for aging for 6 hours, then raise the temperature to 80°C and start distilling ammonia until pH = 6- At 7 o'clock, the obtained precipitate was filtered and washed, and dried at 80° C. for 12 hours. The obtained solid was the copper-silicon material used as the ion-exchange substrate.
[0046] Subsequently, the above 2.4g of the obtained solid, 5.1g of cobalt nitrate hexahydrate, 9.8mL of ammonia water, 3.5g of ammonium chloride, and 0.9g of hydrazine hydrate were dissolved in 200mL of deionized water, stirred continuously at 80°C, and reacted for 6h. The precipitate was centrifuged and washed with a mixture of water and ethanol, dried and air-calcined in a muffle furnace at 500 °C for 4 h.
[0047] Catalyst on-line redu...
Embodiment 2-10
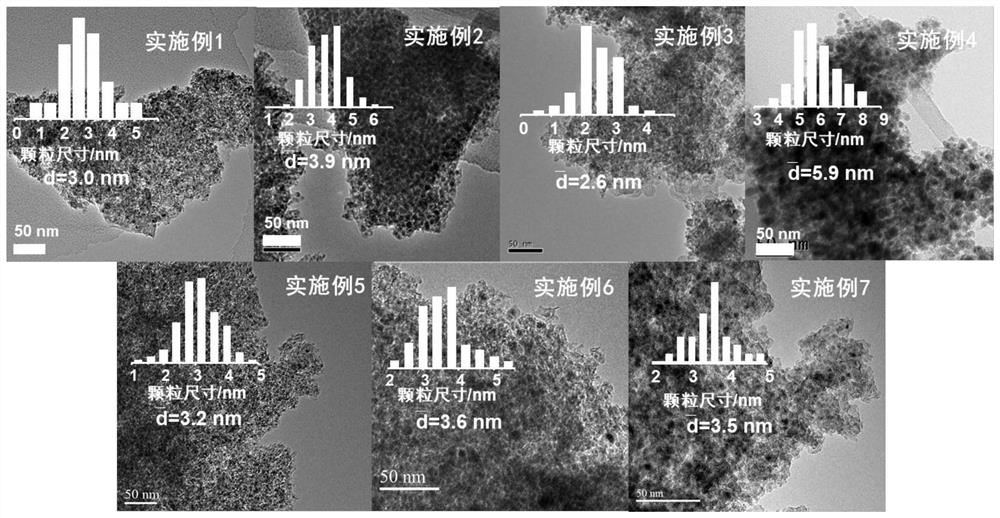
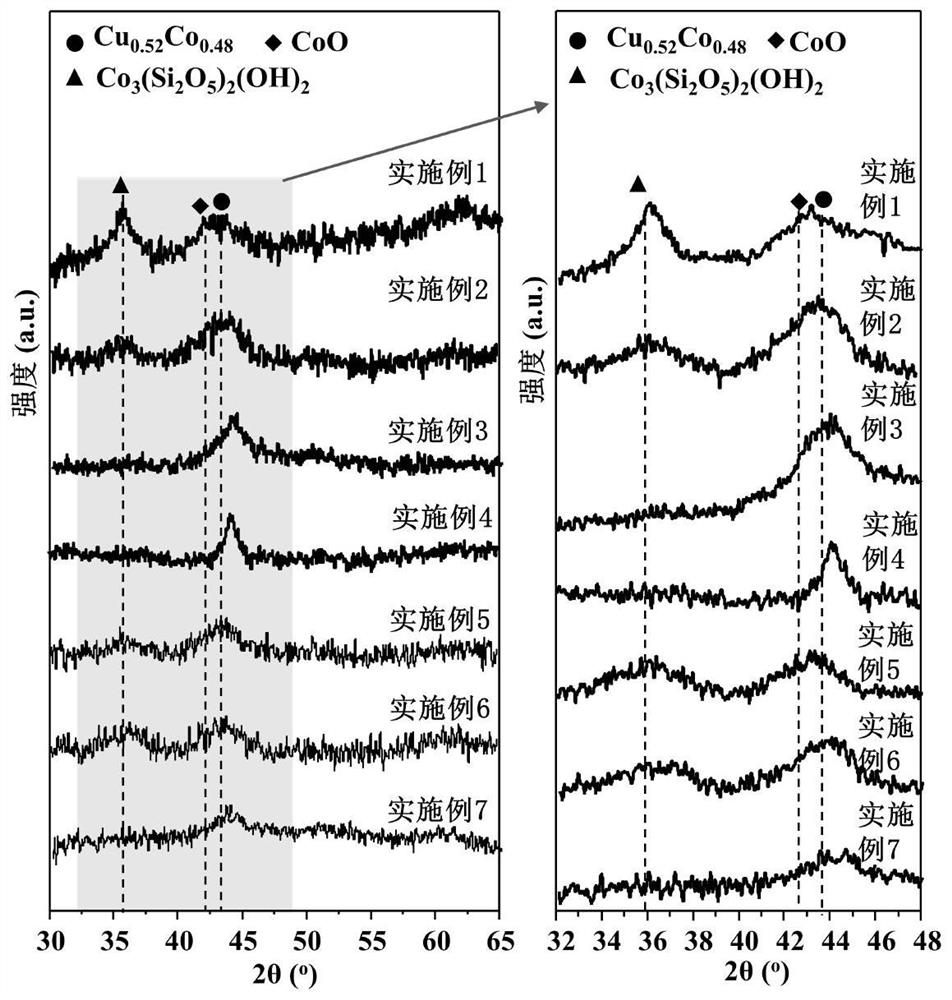
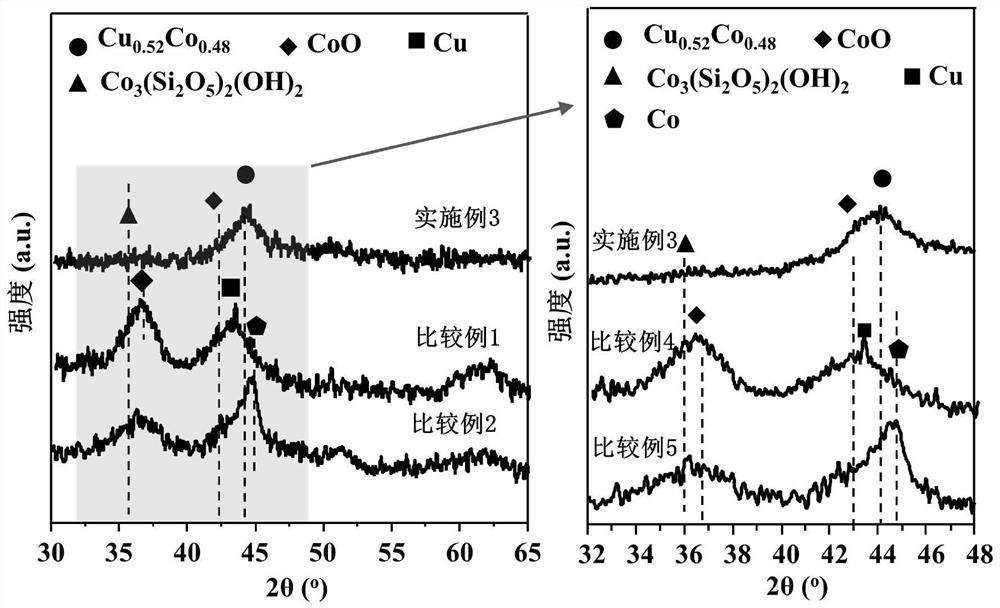
[0051] The preparation method of embodiment 2-10 and the steps of on-line reduction process are consistent with embodiment 1, and the specific parameters are shown in table 1, wherein embodiment 2-4 changes the Co / Cu ratio, and embodiment 5-7 changes the catalyst on-line reduction temperature. See Table 2 for the performance evaluation results of catalysts in various examples in the reaction of syngas to low-carbon alcohols.
[0052] The preparation of table 1 embodiment 2-7 and the process parameter of on-line reduction
[0053]
[0054] The catalytic performance evaluation result of table 2 embodiment 1-10
[0055]
[0056] The evaluation results of the catalysts of Examples 1-10 are shown in Table 2. It can be seen that the CoCu catalysts derived from layered double metal silicate precursors in the present invention perform well when applied to the reaction of producing low-carbon alcohols from synthesis gas. Excellent reactivity and low alcohol selectivity.
[005...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com