Nucleoside compound for antiviral therapy and application thereof
A nucleoside and anti-virus technology, which is applied in anti-viral agents, compounds of group 5/15 elements of the periodic table, organic chemistry, etc., can solve problems such as discounting of immune effects, loss of aquaculture, and impact on the immune protection effect of vaccines , to achieve the effect of novel structure, excellent effect and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
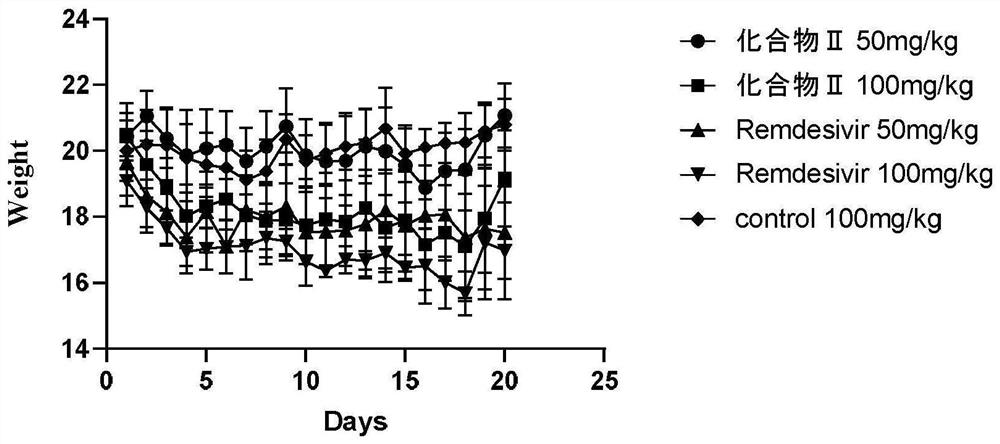
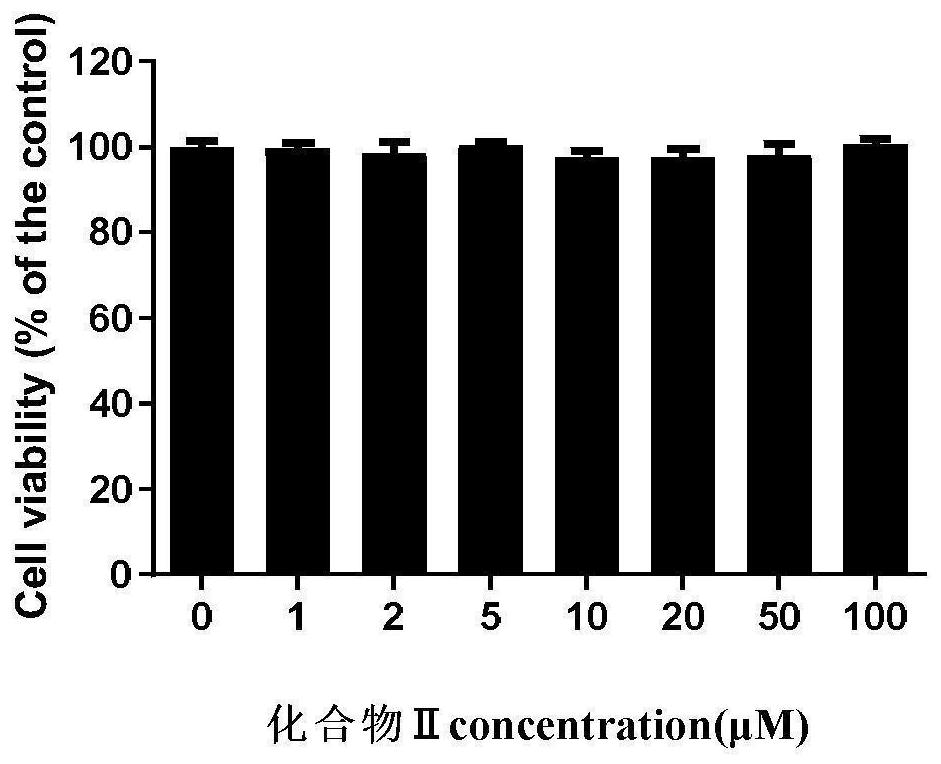
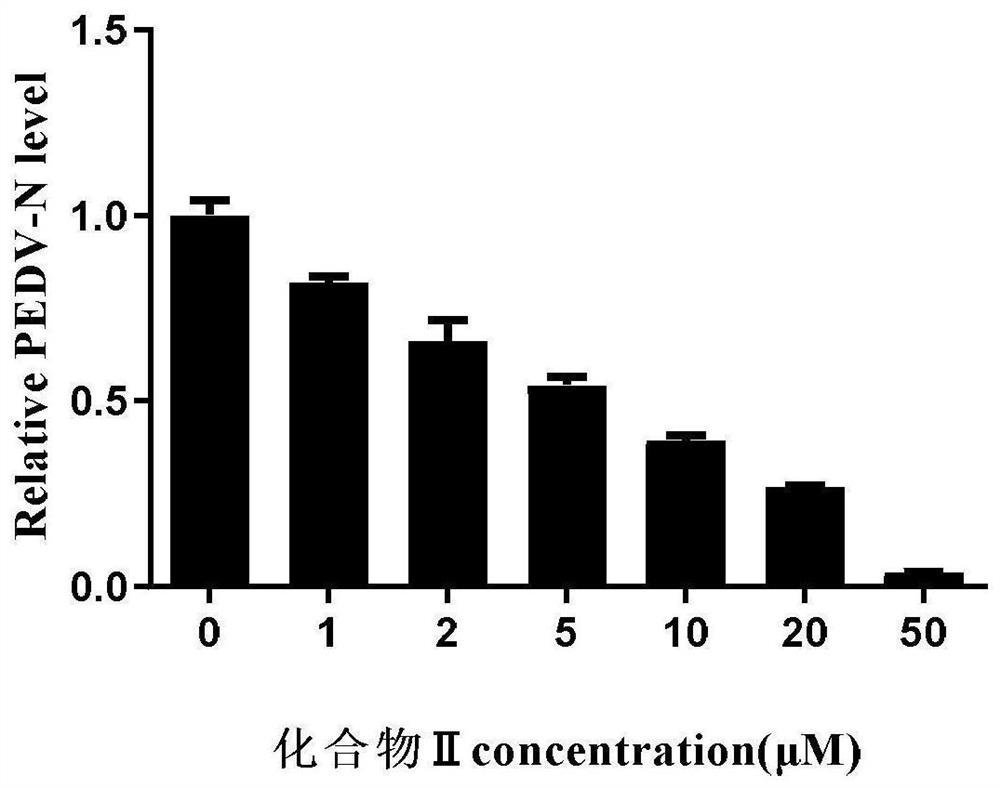
Image
Examples
Embodiment 1
[0037]
[0038] step 1
[0039] Weighed NaOH (985mg, 24.63mmol), added 13mL of water to prepare a solution and placed it in an ice bath with stirring and cooling for 5 minutes, then added bis(N,N-dimethylamino)phosphonyl chloride (2g, 11.73mmol), then raised to room temperature and stirred until the reaction was complete. Then spin the solvent to dry, add ethanol to dissolve, and concentrate the filtrate after suction filtration to obtain compound 1.
[0040] step 2
[0041] Weigh compound 2 (100mg, 0.34mmol) and compound 1 (60mg, 0.34mmol), dimethyl tin dichloride (8mg, 0.034mmol) and place in an eggplant-shaped bottle, add 3mL of N-methylpyrrolidone to dissolve it Dissolved and placed in an oil bath at 80° C. to react overnight until complete. After concentrating the solvent, compound I (96 mg, yield 80%) was prepared by high pressure.
[0042] 1 H NMR (400MHz, DMSO-d 6 )δ8.01(d, J=52.0Hz, 3H), 6.93(d, J=7.6Hz, 2H), 5.24(s, 1H), 4.88-4.61(m, 2H), 4.61-4.43(m, 1H ), ...
Embodiment 2
[0045]
[0046] step 1
[0047] Weigh NaOH (247mg, 6.16mmol), add 3.5mL of water to prepare a solution and place it in an ice bath with stirring and cooling for 5 minutes, then add bis(N,N-dimethylamino)phosphonyl chloride (500mg , 2.93mmol), then raised to room temperature and stirred until the reaction was complete. Then spin the solvent to dry, add ethanol to dissolve, and concentrate the filtrate after suction filtration to obtain compound 1.
[0048] step 2
[0049] Weigh compound 3 (200mg, 0.33mmol) and compound 1 (116mg, 0.66mmol), dimethyl tin dichloride (8mg, 0.033mmol) and place in an eggplant-shaped bottle, add 4mL of N-methylpyrrolidone to dissolve it Dissolved and placed in an oil bath at 80° C. to react overnight until complete. After concentrating the solvent, compound II (171 mg, yield 78%) was prepared by high pressure.
[0050] 1 H NMR (400MHz, DMSO-d 6 )δ8.19(s, 2H), 7.97(d, J=7.1Hz, 1H), 7.32(t, J=8.1Hz, 2H), 7.17(t, J=7.4Hz, 3H), 7.05-6.79( m, 2H)...
Embodiment 3
[0053]
[0054] step 1
[0055] Weigh compound I (2057mg, 5.81mmol) and place it in an eggplant-shaped bottle, then add 24mL triethylamine to dissolve the substrate completely, then add 4-dimethylaminopyridine (36mg, 0.29mmol) and place the reaction system in Stir for 5-10 minutes under ice bath. Isobutyric anhydride (1011 mg, 6.39 mmol) was then added slowly. Warmed to room temperature, the reaction mixture was stirred until the reaction was complete. Concentrate the reaction mixture by distilling off the solvent under reduced pressure, add the residue to water, extract with ethyl acetate and saturated sodium bicarbonate, wash the organic layer with saturated brine, dry with anhydrous sodium sulfate, and distill off the solvent under reduced pressure . The resulting residue was purified by silica gel column chromatography (dichloromethane-methanol) to obtain compound III (1536 mg, yield 62.5%).
[0056] 1 H NMR (400MHz, DMSO-d 6 )δ8.01(d, J=52.0Hz, 3H), 6.93(d, J=7.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com