Canagliflozin impurities, and preparation and removal methods thereof
A technology for process impurities and intermediates, applied in chemical instruments and methods, preparation of sugar derivatives, organic chemistry methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation of formula I compound
[0043] Take canagliflozin intermediate (Ia) and refine 900mL of mother liquor with isopropyl acetate, concentrate at 45°C under reduced pressure (-0.08~-0.1MPa), add 9.5g of silica gel when it is concentrated to about 70mL, and continue to concentrate to obtain Silicone adsorbent.
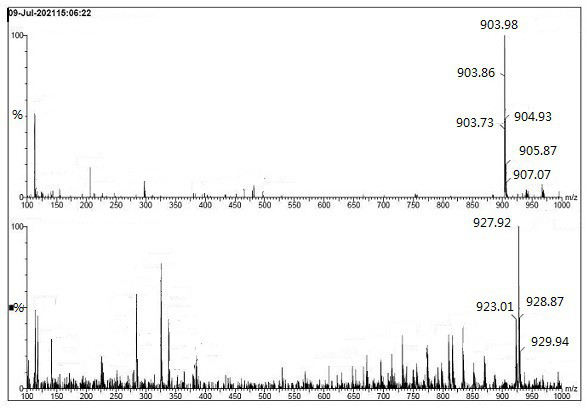
[0044] The above silica gel adsorbate was separated by column chromatography, eluent: V dichloromethane / methanol=30 / 1 (1L); V dichloromethane / methanol=25 / 1 (2L); V dichloromethane / methanol =20 / 1 (1L); V dichloromethane / methanol=10 / 1 (1L); V dichloromethane / methanol=1 / 1 (1L). Collect the eluent containing the target compound (TLC developer: V dichloromethane / methanol = 10 / 1, Rf target compound = 0.3, Rf canagliflozin = 0.5). Concentrate the eluent to obtain 0.21 g of off-white solid, which is the compound of formula I.
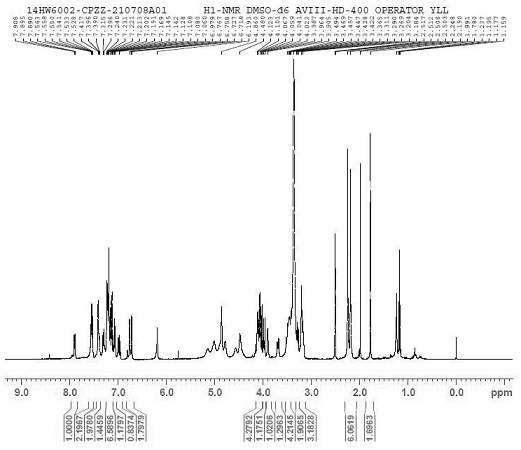
[0045] [1H NMR (400 MHz, DMSO-d6) δ 7.90 (dd, J = 7.5, 2.5 Hz, 1H), 7.55(ddd, J = 8.7, 5.6, 2.8 Hz, 2H), 7.44 – 7.37 (m, 2H) , 7.3...
Embodiment 2
[0047] The preparation of formula II compound
[0048] Get canagliflozin impurity formula I (500mg, 0.55mmol), add 20mL dichloromethane to dissolve therein, then add 4-dimethylaminopyridine (29mg, 0.24mmol), add dropwise acetic anhydride (560mg, 5.50mmol), React at room temperature (20~30°C) for 2h, add 20mL of water, separate the liquid, collect the organic phase, wash with water twice, concentrate under reduced pressure (-0.08~-0.1MPa) at 45°C to obtain an oily concentrate, and pass column chromatography Purified by the method, eluent (V petroleum ether / ethyl acetate=2 / 1), collected the eluent containing the target compound, concentrated to dryness under reduced pressure (-0.08~-0.1MPa) at 45°C, and obtained 0.42 g Yellow solid (Formula II).
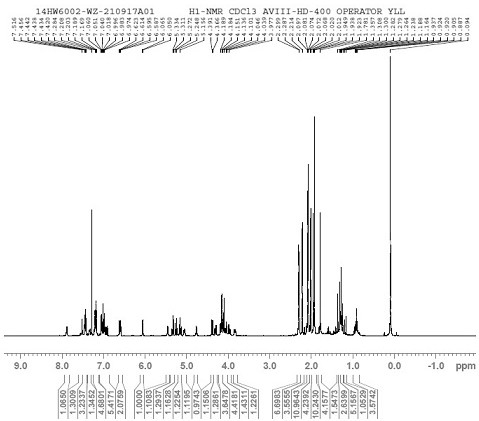
[0049] [1H NMR (400 MHz, Chloroform-d) δ 7.88 (dd, J = 7.4, 2.4 Hz, 1H),7.52 (d, J = 2.0 Hz, 1H), 7.44 (ddt, J = 7.0, 5.2, 2.5 Hz , 3H), 7.23 – 7.15(m, 4H), 7.07 – 7.03 (m, 1H), 7.03 – 7.01 (m, 1H), 6.98 (d, J = 3.6 Hz, 1H), 6.92 (dd...
Embodiment 3
[0051] Removal of compounds of formula II
[0052] Add 5.0g of canagliflozin intermediate I-Ac, 10mL of ethyl acetate into a 50mL reaction bottle, heat up to 65~75°C, dissolve, then cool down to 20~30°C, add 10mL of tertiary methyl ether, 0.3 mL of water , after stirring for 2h, further lower the temperature to 0~5°C, stir and crystallize at 0~5°C for 1h, filter, rinse with an appropriate amount of tertiary methyl ether, and blow dry at 45°C to obtain 4.11g of refined canagliflozin intermediate I-Ac , yield 82.2%, HPLC detection purity 99.91%, formula II impurity content 0.09% (HPLC detection purity before refining 99.17%, impurity content 0.62%), formula II impurity removal rate 85.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com