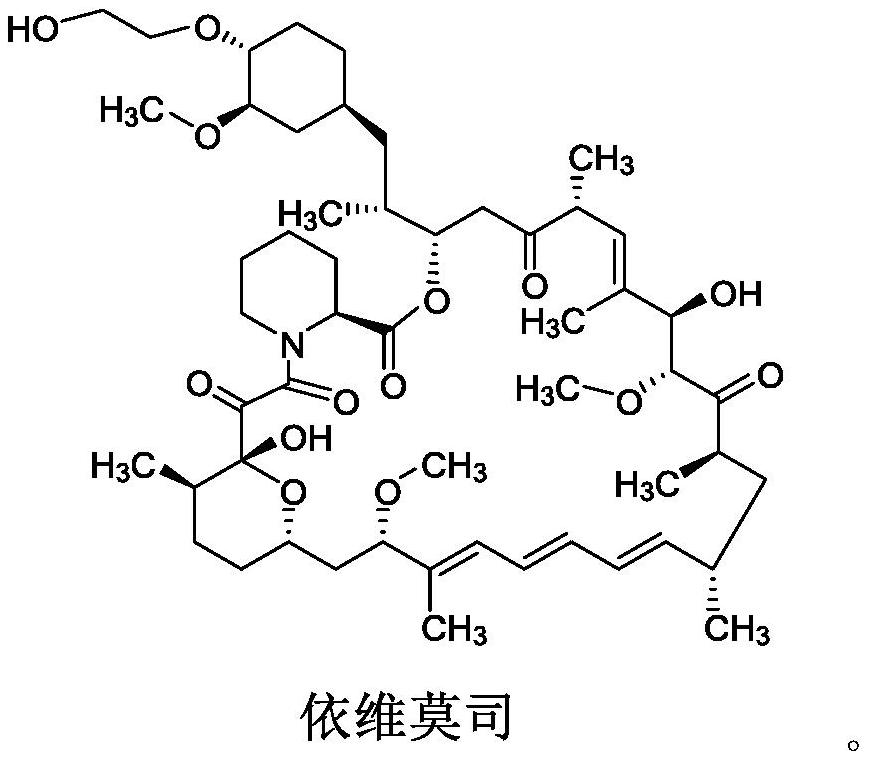
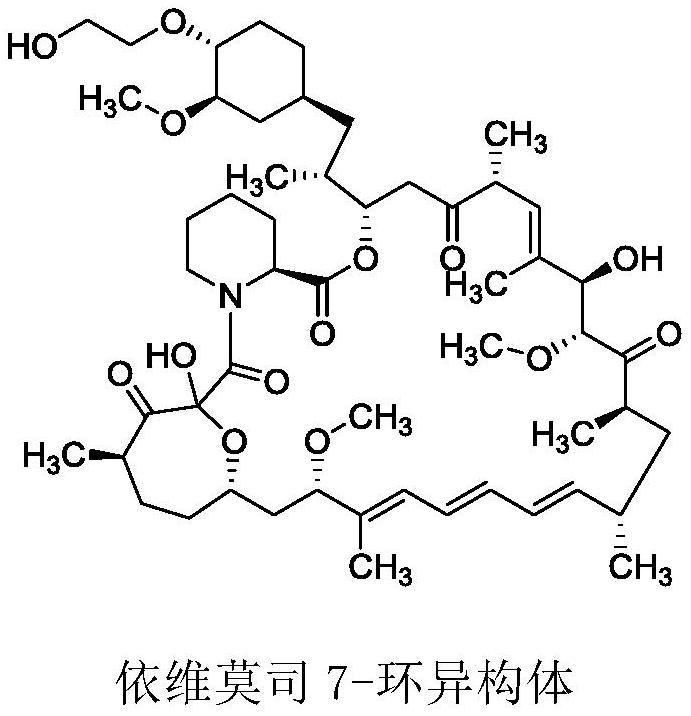
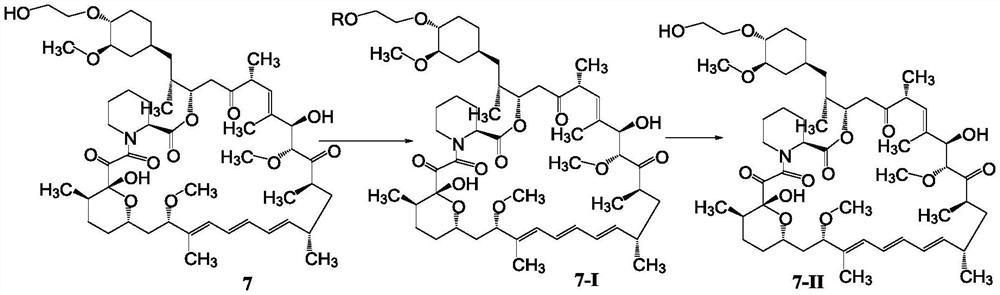
A kind of purification method of everolimus
A purification method, everolimus technology, applied in the field of organic synthesis, can solve the problems of high cost and unfavorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1: the preparation of compound 2
[0067]
[0068] Ethyl acetate (1.7 L) and rapamycin (140 g, compound 1) were sequentially added into the reaction flask and stirred to dissolve. Add imidazole (47g), lower the temperature to 0-5°C, start to add TMS-Cl (66.5g) dropwise, after dropping, control the reaction at 0-5°C for 0.5h, monitor by TLC until the reaction of raw materials is complete. Adjust dilute sulfuric acid to pH = 1, continue the reaction for 2.5 h, adjust to pH = 7 with imidazole, wash with saline, dry over anhydrous sodium sulfate, filter, concentrate the filtrate, and the concentrate is subjected to silica gel column chromatography (mobile phase conditions: ethyl acetate Ester / n-hexane gradient elution) to obtain off-white compound 2 (yield: 91%).
Embodiment 2
[0069] Embodiment 2: the preparation of compound 6
[0070]
[0071] 2,6-lutidine (72g), dichloromethane (320ml) and compound 3 (98.7g) were added to the reaction flask in sequence, and stirred evenly. Control temperature at -5~5℃ and add Tf dropwise 2 O / dichloromethane solution (174g / 95ml), dropwise until compound 3 reacts completely. Washed with water, dried over anhydrous sodium sulfate, filtered, concentrated, the concentrate (compound 4, 172.6g) was dissolved in n-hexane (690ml), and 2,6-di-tert-butyl-4-methylpyridine (115g) and Compound 5 (138g), stirred at 50-60°C, reacted for 22h, and the reaction was complete. Purified by flash silica gel column and concentrated to obtain off-white compound 6 (yield 70%).
[0072] 13 C-NMR (CDCl 3 ,500MHz)δ:209.1,207.1,198.6,169.1,166.9,139.0,137.8,136.3,132.2,130.3,125.4,126.9,126.8,98.9,85.0,82.6,77.5,73.6,70.7,66.1,96.5, ,55.3,50.9,44.9,43.5,40.6,40.2,40.1,39.0,37.9,35.8,35.0,34.7,33.5,32.4,32.3,31.1,29.7,26.3,26.2,25.7,24...
Embodiment 3
[0074] Embodiment 3: Preparation of Everolimus 7
[0075]
[0076] Acetonitrile (220 mL) and compound 6 (22 g) were sequentially added into a 500 mL reaction flask, and stirred to dissolve. Slowly add 0.2mol / L hydrochloric acid (19.2ml) under temperature control at -10-0°C, and react for 0.5h after addition, and monitor by TLC until the reaction is complete. Add dichloromethane / purified water (220mL / 220mL) while controlling the temperature at -10-0°C, stir and separate the layers, and extract the aqueous phase with dichloromethane (106mL). The organic phases were combined, washed with purified water (220 mL×3), dried over anhydrous sodium sulfate, filtered, and concentrated to dryness to obtain solid everolimus 7 (20.4 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com