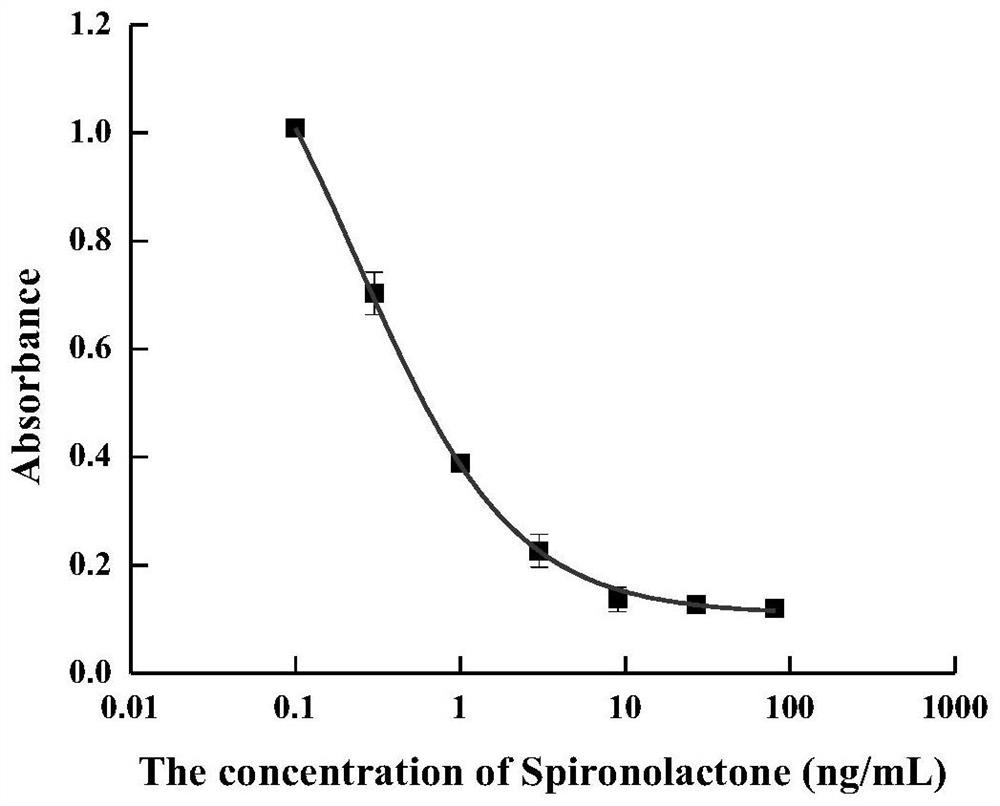
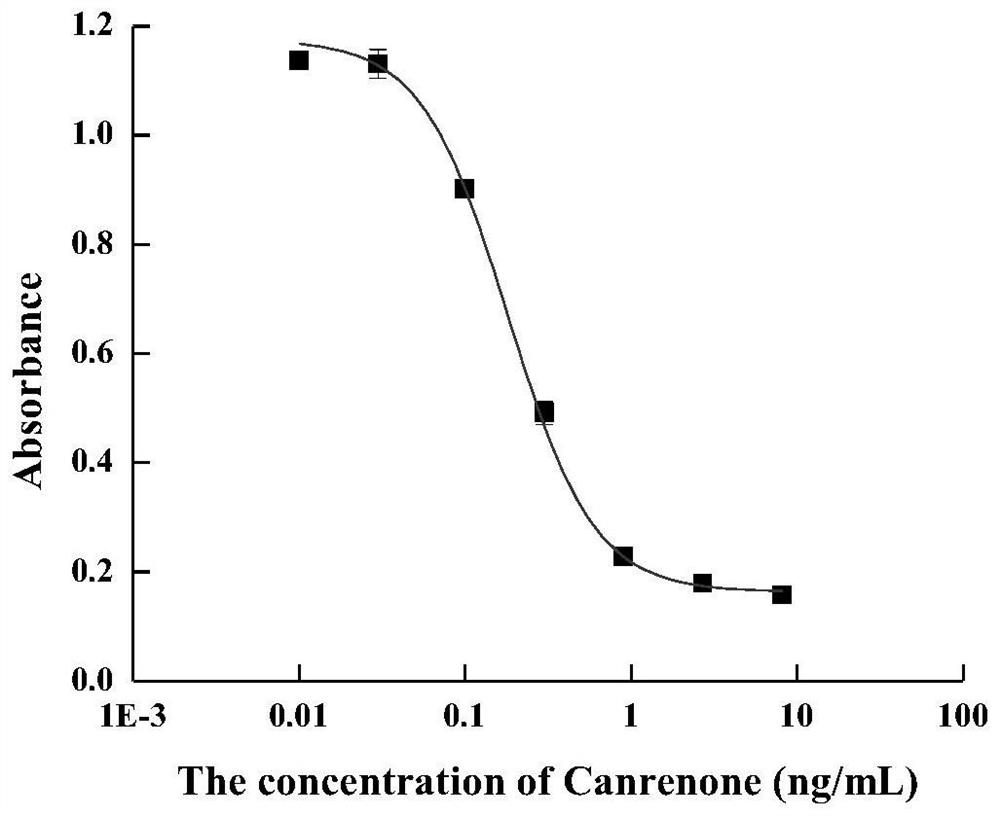
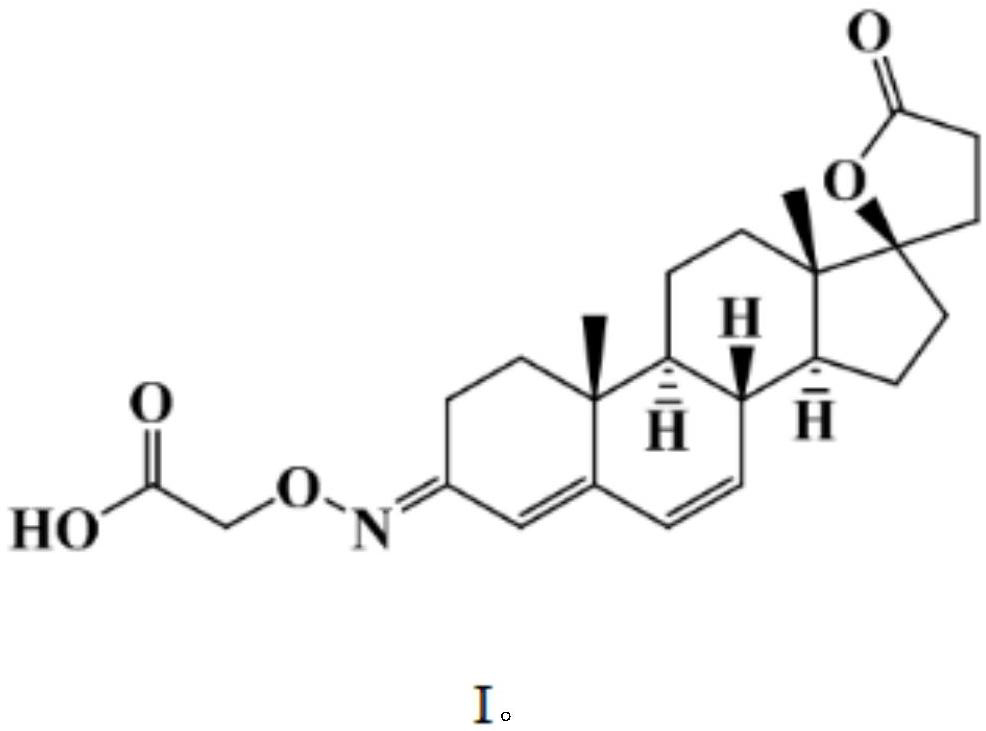
Hybridoma cell strain for secreting spirolactone and metabolite monoclonal antibody thereof and application of hybridoma cell strain
A hybridoma cell line, monoclonal antibody technology, applied in microorganisms, analytical materials, biological tests, etc., can solve the problems of time-consuming, high cost, and complicated steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Synthesis of embodiment 1 spironolactone and its metabolite hapten
[0050] Dissolve 1 g of spironolactone (or canrenone) and 3 g of CMO in 10 mL of DMF, and heat in a water bath at 60° C. for 8 h. The organic solvent was removed by rotary evaporation under reduced pressure to obtain a yellow precipitate. Wash three times with deionized water and 1M HCl, add ethanol for recrystallization, and obtain light yellow or white needle-like crystals, namely the hapten Spi-COOH (or Can-COOH) of spironolactone and its metabolites.
[0051] The reaction equation is as follows:
[0052]
Embodiment 2
[0053] The synthesis of embodiment 2 spironolactone and its metabolite complete antigen
[0054] (1) Preparation of the immunogen spironolactone and its metabolite-BSA: Weigh 3.4mg of prepared Can-COOH, dissolve it in 200μL DMF, add 4.6mg N-hydroxysuccinimide and 7.7mg 1- Ethylcarbodiimide hydrochloride, react at room temperature for 4 hours, and the resulting mixture is called solution A; then weigh 10 mg of bovine serum albumin BSA and dissolve it in 2 mL of carbonate buffer, called solution B; slowly drop solution A Pour into solution B, react at room temperature for 12 hours under stirring, and dialyze with 0.01mol / L phosphate buffer solution PBS for 3 days to obtain the conjugated spironolactone and its metabolite -BSA, which should be frozen at -20°C for later use;
[0055] (2) Preparation of coated prospironolactone and its metabolite-OVA: Weigh 1.7mg of the prepared Spi-COOH, dissolve it in 200μL of DMF, add 2.3mg of N-hydroxysuccinimide and 3.4mg of 1 - Ethylcarbodii...
Embodiment 3
[0056] Example 3 Preparation of hybridoma cell lines secreting spironolactone and its metabolite monoclonal antibody
[0057] 1. Immunization of mice: healthy BALB / c mice aged 6-8 weeks were selected for immunization. The complete antigen of spironolactone and its metabolite was mixed and emulsified with an equal amount of Freund's adjuvant, and BALB / c mice were immunized by subcutaneous injection in the back. Complete Freund's adjuvant was used for the first immunization, followed by incomplete Freund's adjuvant. The interval between the first immunization and the second booster immunization was 28 days, and the interval between multiple booster immunizations was 21 days. Blood was collected 7 days after the third immunization (5 μL of mouse tail blood collection + 995 μL antibody diluent = antiserum), and ic-ELISA was used to measure the titer and inhibition of the mouse serum, and the mice with high titer and inhibition were selected, and the mice with high titer and inhib...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com