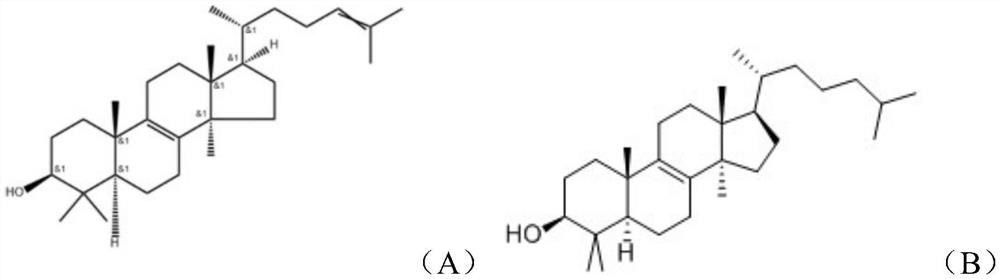
Method for separating lanosterol and dihydrolanosterol
A technology of lanosterol and dihydrogoat, applied in steroids, organic chemistry and other directions, can solve the problem of large amount of solvent used, and achieve the effect of reducing the amount of solvent, high yield and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Weigh 30g of the raw material and 30g of the protic solvent methanol in a volumetric flask to make a raw material solution at a ratio of 1:1. Using calcium chloride as a complexing agent, weigh 8.5 g of calcium chloride and add it to the above raw material solution, stir and react at 60° C. and 350 r / min for 1 hour, cool, and filter to obtain a complex. Dissolve the complex with a certain amount of water and organic matter, choose ethyl acetate as the organic matter, stir at 40°C for 30 minutes, separate the organic layer, and evaporate to dryness by rotary evaporation to obtain the crude product of lanosterol, and then obtain the pure product of lanosterol through solvent crystallization 13.2g, its product purity is 95.7%, and the yield reaches 78.5%. The complex mother liquor was crystallized by solvent at 60°C to obtain 10.5 g of pure dihydrolanosterol with a purity of 90.1% and a yield of 95.7%.
Embodiment 2
[0028] Weigh 30g of raw materials and 60g of methanol in a volumetric flask to make a raw material solution at a ratio of 1:2, use calcium chloride as a complexing agent, weigh 8.5g of calcium chloride and add it to the above raw material solution, and mix it under the conditions of 60°C and 350r / min The reaction was stirred for 1h, cooled and filtered to obtain the complex. Dissolve the complex with a certain amount of water and organic matter, choose ethyl acetate as the organic matter, stir at 40°C for 30 minutes, separate the organic layer, and evaporate to dryness by rotary evaporation to obtain the crude product of lanosterol, and then obtain the pure product of lanosterol through solvent crystallization 12g, its product purity is 93.5%, and the yield reaches 69.3%. The complexed mother liquor was crystallized by solvent at 60°C to obtain 10 g of pure dihydrolanosterol with a purity of 86.4% and a yield of 90.3%.
Embodiment 3
[0030] Weigh 60g of raw material and 30g of methanol in a volumetric flask to make a raw material solution at a ratio of 2:1, use calcium chloride as a complexing agent, weigh 8.5g of calcium chloride and add it to the above raw material solution, at 60°C and 350r / min The reaction was stirred for 1h, cooled and filtered to obtain the complex. Dissolve the complex with a certain amount of water and organic matter, choose ethyl acetate as the organic matter, stir at 40°C for 30 minutes, separate the organic layer, and evaporate to dryness by rotary evaporation to obtain the crude product of lanosterol, and then obtain the pure product of lanosterol through solvent crystallization 23.5g, its product purity is 94.9%, and the yield reaches 70.4%. The complexed mother liquor was crystallized by solvent at 60°C to obtain 20.2 g of pure dihydrolanosterol with a purity of 87.4% and a yield of 91.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com