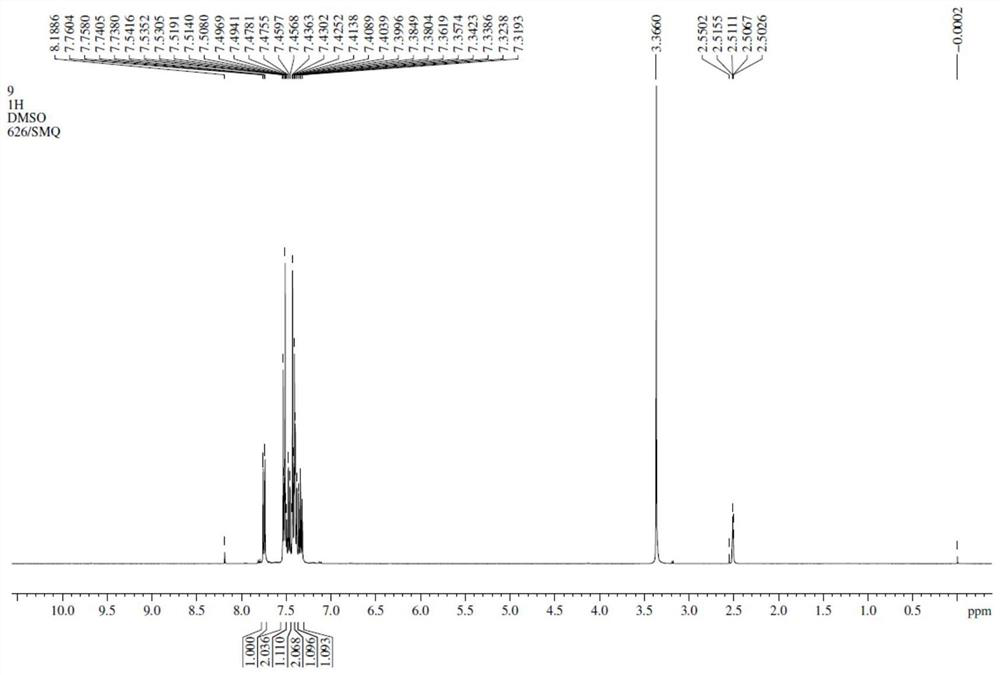
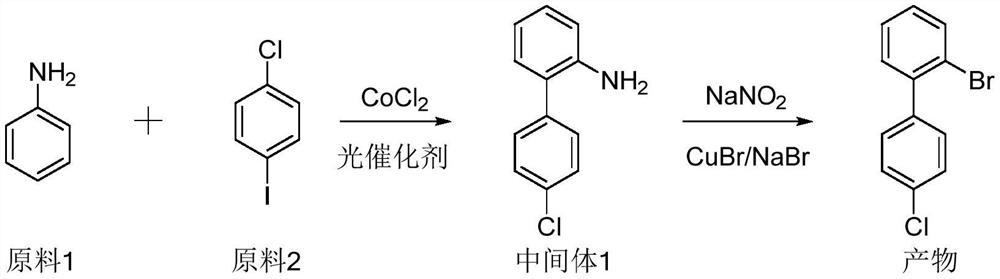
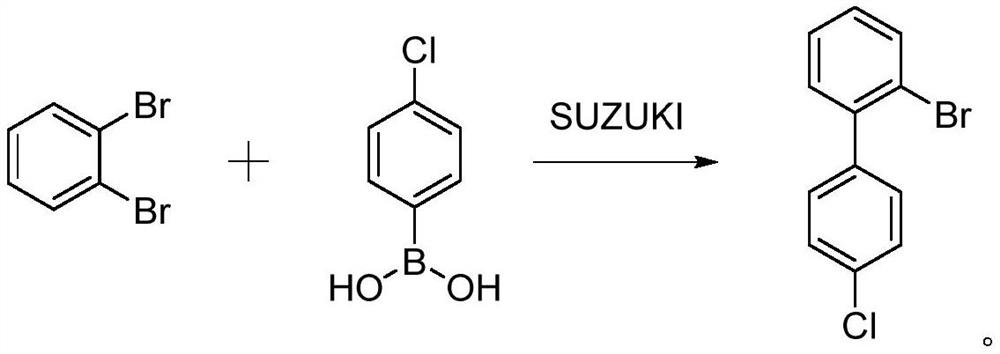
Synthesis method of 2-bromo-4 '-chloro-1, 1'-biphenyl
A synthesis method and biphenyl technology are applied in the field of synthesis of 2-bromo-4'-chloro-1,1'-biphenyl, and can solve the problems of high price, poor coupling reaction selectivity, difficulty in purification and separation, and the like, and achieve The effect of saving production cost, mild reaction conditions and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] This example provides a synthesis method of 2-bromo-4'-chloro-1,1'-biphenyl, the synthesis process is as follows:
[0027] (1) Synthesis of intermediate 1
[0028] Aniline (9.3g, 0.1mol) and p-chloroiodobenzene (28.6g, 0.12mol) were added to a mixed solvent of acetonitrile and water (90mL / 10mL), and then cobalt chloride (CoCl 2 ) (0.26g, 0.002mol), fluorescein (2.65g, 0.008mol), and finally the reaction was irradiated with 525nM LED green light at 70°C for 21 hours. TLC monitors that the aniline reaction is complete, then cool down, add 250mL of ethyl acetate, wash with saturated aqueous sodium chloride solution, combine the organic phases, dry and spin dry, and the obtained crude product is dissolved in a small amount of ethyl acetate, and then beaten in petroleum ether to obtain The product of intermediate 1 is 18.4 g in total, and the yield is 90.4%.
[0029] (2) Synthesis of product 2-bromo-4'-chloro-1,1'-biphenyl
[0030] Dissolve Intermediate 1 (2.0g, 0.01mol) ...
Embodiment 2
[0032] This example provides a synthesis method of 2-bromo-4'-chloro-1,1'-biphenyl, the synthesis process is as follows:
[0033] (1) Synthesis of intermediate 1
[0034] Aniline (9.3g, 0.1mol) and p-chloroiodobenzene (28.6g, 0.12mol) were added to a mixed solvent of acetonitrile and water (90mL / 10mL), and then cobalt chloride (CoCl 2 ) (0.26g, 0.002mol), rhodamine (3.8g, 0.008mol), and finally the reaction was irradiated with 525nM LED green light at 70°C for 36 hours. TLC monitors that the aniline reaction is complete, then cool down, add 250mL of ethyl acetate, wash with saturated aqueous sodium chloride solution, combine the organic phases, dry and spin dry, and the obtained crude product is dissolved in a small amount of ethyl acetate, and then beaten in petroleum ether to obtain The product of intermediate 1 is 16.6g in total, and the yield is 81.6%.
[0035] (2) Synthesis of product 2-bromo-4'-chloro-1,1'-biphenyl
[0036] Intermediate 1 (2.0g, 0.01mol) was dissolved...
Embodiment 3
[0038] This example provides a synthesis method of 2-bromo-4'-chloro-1,1'-biphenyl, the synthesis process is as follows:
[0039] (1) Synthesis of intermediate 1
[0040] Aniline (930g, 10mol) and p-chloroiodobenzene (2860g, 12mol) were added to a mixed solvent of acetonitrile and water (9L / 1L), and then cobalt chloride (CoCl 2 ) (26g, 0.2mol), fluorescein (265g, 0.8mol), and finally the reaction was irradiated with 525nM LED green light at 70°C for 36 hours. TLC monitors that the aniline reaction is complete, then lowers the temperature, adds 20L of ethyl acetate, washes with saturated aqueous sodium chloride solution, combines the organic phases, dries and spins to dry, and dissolves the obtained crude product in ethyl acetate, then beats in petroleum ether to obtain intermediate The product of body 1 is 1821g in total, and the yield is 89.5%.
[0041] (2) Synthesis of product 2-bromo-4'-chloro-1,1'-biphenyl
[0042] Intermediate 1 (200g, 1mol) was dissolved in 0.5mol / L h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com