Packaging body of edaravone solid dispersion or preparation thereof
A technology of solid dispersion and edaravone is applied in the packaging of edaravone solid dispersion or its preparation, and in the field of packaging to improve the stability of edaravone solid dispersion or its preparation, and can solve the problem of total impurities. Increased content, no edaravone solid dispersion or its formulation stability packaging method, affecting stability and product quality, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Dissolve 5 g of Edaravone and 25 g of Soluplus in 173 ml (about 136.7 g) of methanol, and prepare a solid dispersion (ASD) by spray drying and vacuum drying.
Embodiment 2
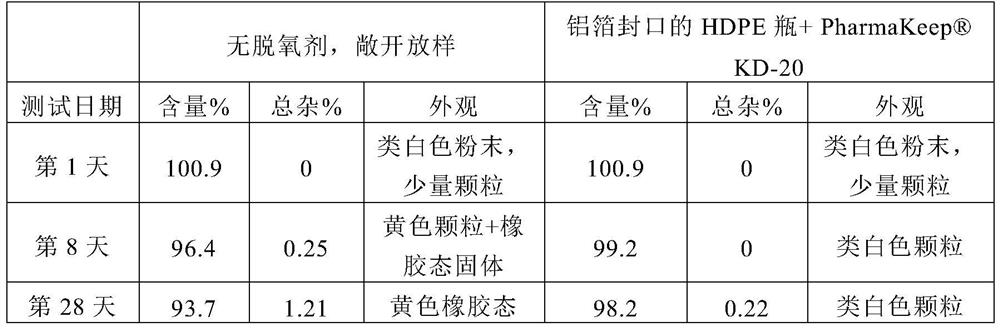
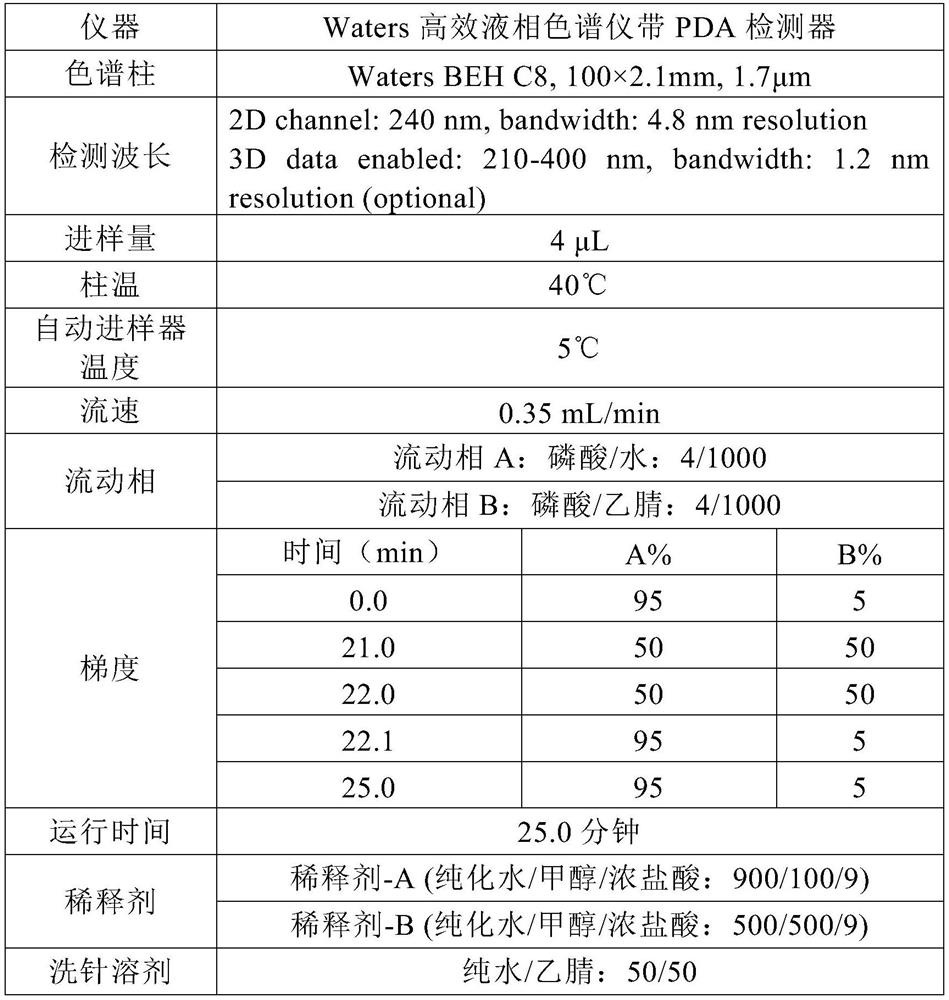
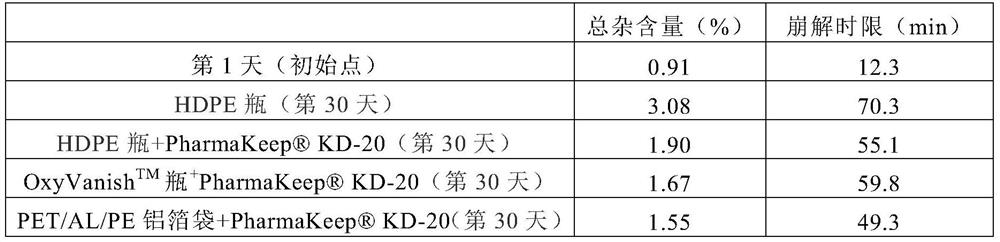
[0039] Dissolve 30g of Edaravone and 150g of Soluplus in 1038ml (about 820g) of methanol, and prepare a solid dispersion by spray drying and vacuum drying. The auxiliary materials used for granulation include 190g of microcrystalline cellulose and 100g of mannitol , 10g sodium carboxymethyl starch, 2.5g silicon dioxide, 2.5g magnesium stearate, mixed, dry powder granulation; additional auxiliary materials for tableting include 12.5g sodium carboxymethyl starch and 2.5g magnesium stearate, Blending and tableting to make 1000 tablets, each weighing 500mg, packaged in HDPE bottles, HDPE bottles + deoxidizer KD-20, OxyVanish TM bottle + Deoxidizer KD-20, PET / AL / PE aluminum foil bag + deoxidizer KD-20.
Embodiment 3
[0041] Dissolve 30g of Edaravone and 150g of Soluplus in 1038ml (about 820g) of methanol, and prepare a solid dispersion by spray drying and vacuum drying. Sodium hydrogen, 160g mannitol, 128g crospovidone, 16g sodium carboxymethyl starch, 4g magnesium stearate, mixed, dry powder granulation; additional excipients for tableting include 32g crospovidone, 24g carboxy Sodium methyl starch and 8g magnesium stearate, mixed together, compressed into 1000 tablets, each weighing 800mg, in PET / AL / PE aluminum foil bag + deoxidizer KD-20 for packing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com