Heat-resistant mannosidase gene as well as expression protein and application thereof
A mannosidase and heat-resistant technology, applied in the field of bioengineering, can solve the problems of low enzymatic hydrolysis efficiency, cost problems, and hard structure of agricultural and sideline products, and achieve the effect of great superiority, strong heat resistance, and single product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 mannosidase gene
[0030] Using the amino acid sequence of Pseudothermotoga thermarum DSM 5069 mannosidase (NCBI number: AEH51527.1) as a template, the non-conserved site of mannosidase was obtained as a potential transformation site through database comparison; the three-dimensional site after saturation mutation was simulated by computer structure, use AutoDock to simulate the interaction between mannose and protein, and select the optimal modification scheme. The modified sites are shown in Table 1. The modified amino acid sequence was codon-optimized according to the codon preference of Escherichia coli, and the gene was artificially synthesized. The gene sequence is shown in SEQ NO:1.
[0031]
[0032]
[0033] Table 1 Amino acid modification sites
Embodiment 2
[0034] Construction and verification of embodiment 2 recombinant cloning, expression vector pET-28a-PtMan
[0035] The purified PCR product (prepared in Example 1) and pET-28a (Novagen) were double-digested with NdeI and XhoI respectively, and agarose electrophoresis was used to recover the enzyme-cut PCR and large vector fragments. Add 1 μL of 10X Ligase Buffer and 1 μL of Ligase to the target fragment recovered by tapping the gel and connect it overnight at 16°C. Escherichia coli DH5α was transformed with the product of the ligation reaction, and then spread on a petri dish containing 100 μg / mL Kana (Kanapenicillin), and incubated at 37° C. for 10-15 hours.
[0036] Pick multiple single colonies from the transformation plate, and use BIOMIGA's plasmid mini-extraction kit to extract the plasmid. The obtained plasmid was verified by double enzyme digestion and the obtained recombinant plasmid was sequenced. Sequencing results showed that the cloned target fragment (3033bp in...
Embodiment 3
[0037] Embodiment 3 Expression and purification of recombinant mannosidase
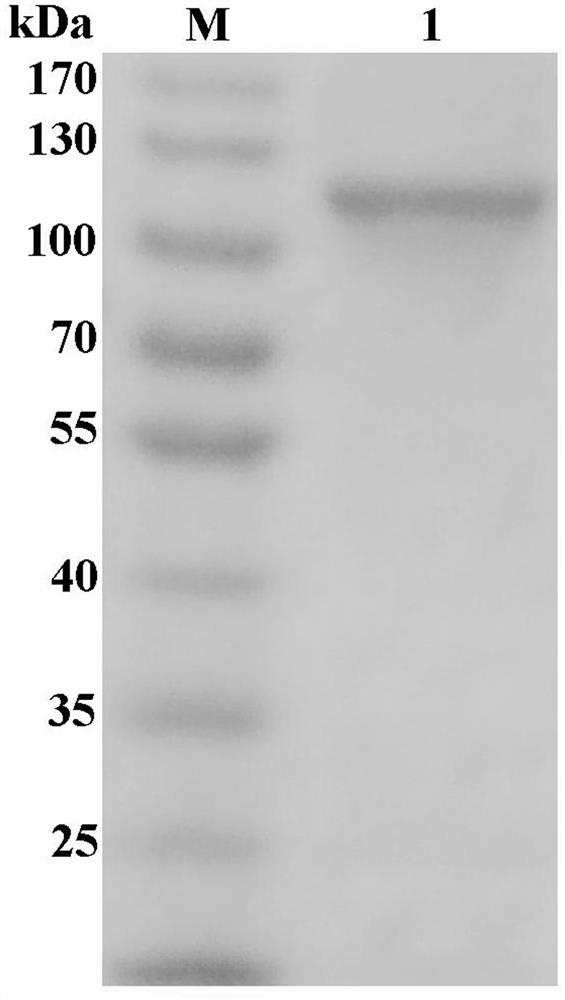
[0038] The recombinant clone and expression vector pET-28a-PtMan (prepared in Example 2) were heat-shock transformed into the host bacterium E.coliBL21 (DE3) (Novagen) to obtain the recombinant bacterium containing the recombinant plasmid. A single colony of recombinant bacteria was inoculated into 5 mL of Luria-Bertani broth (LB) medium containing 100 μg / mL kanapenicillin, and cultured at 37° C. with shaking at 200 rpm for 4 h. Inoculate the above 4mL bacterial solution into a 2000mL shake flask containing 800mL of culture medium, shake at 37°C at 200rpm, and when the absorbance reaches 0.4-0.6, add 800μL of 0.1M IPTG, and incubate at 22°C at 150rpm Induce expression for 15h. The culture solution was centrifuged at 6000 rpm for 10 min at 4°C with a high-speed refrigerated centrifuge to collect the bacteria. Wash with 50mL ultrapure water and centrifuge at 6000rpm at 4°C for 10min, recover the bacte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com