Preparation method of cobalt-coated cobalt-aluminum hydroxide supercapacitor material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 11.7g of cobalt chloride hexahydrate (CoCl 2 ·6H 2 O) and 3.75g aluminum chloride hexahydrate (AlCl 3 ·6H 2 O) Mix (the molar ratio of cobalt to aluminum is 4:1), and add 20.0g of hexamethylenetetramine into 250mL of distilled water at room temperature; then, under rapid stirring, keep warm at 120°C for 2 days (48h) , the precipitation reaction fully occurred; after the reaction was completed, it was filtered, washed three times with water and ethanol successively, and dried to obtain 3.80 g of solid (cobalt-aluminum layered hydroxide) with a yield of about 72%.
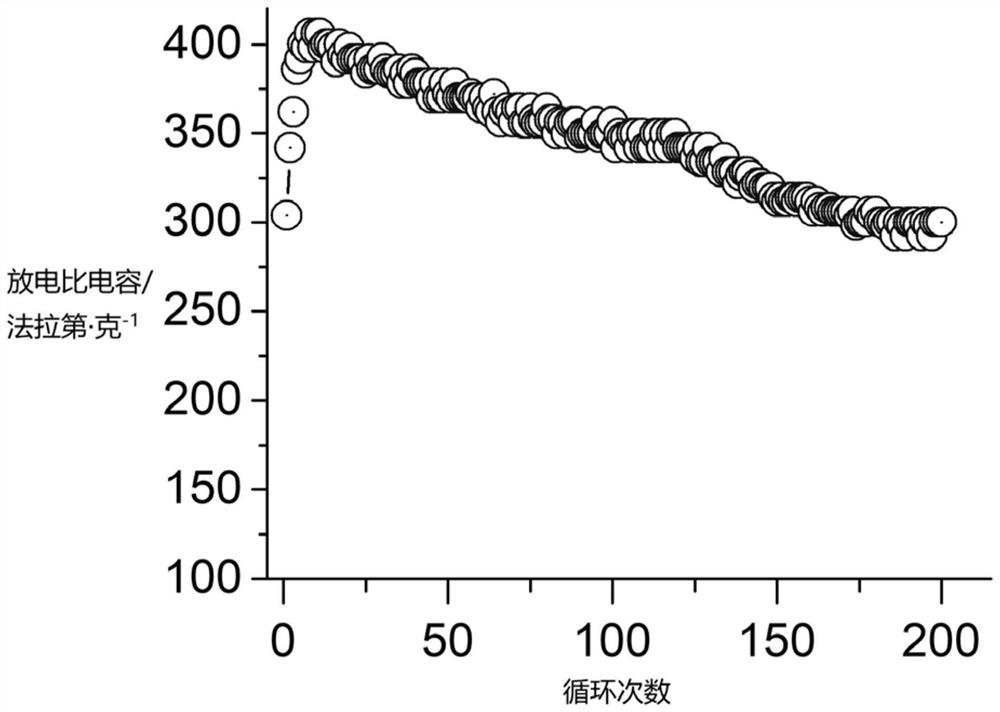
[0037]Take 1.00 g of the solid cobalt-aluminum layered hydroxide prepared above, and add it to 40 mL of potassium hydroxide solution with a concentration of 7 mol / L, then add 0.20 g of polyvinylpyrrolidone and stir evenly; then add 1.00 g of Hexaammine cobalt trichloride, after stirring and dissolving, add 0.50g sodium borohydride, react for 30h, and the reaction temperature is 30°C; after the reaction is c...
Embodiment 2
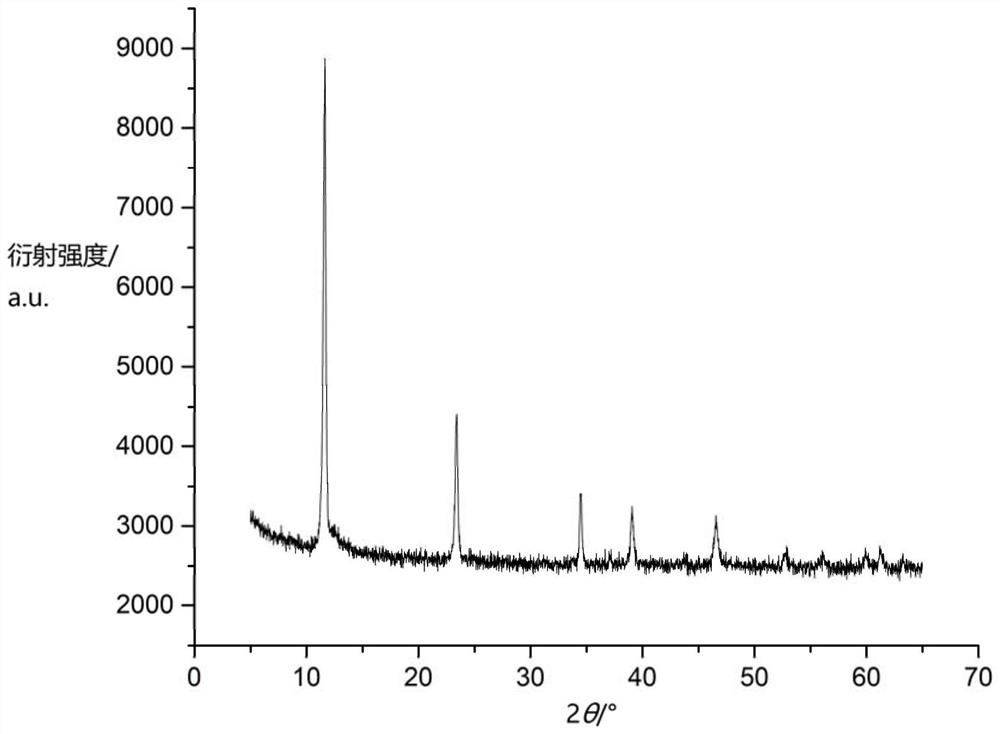
[0043] Dissolve 1.16g of cobalt nitrate, 0.75g of aluminum nitrate (the molar ratio of cobalt to aluminum is 2:1) and 5.0g of urea (urea) in 150mL of distilled water, raise the temperature to 100°C, keep stirring, and keep warm for 10h to fully precipitate Reaction: After the reaction is completed, filter, wash, and dry to obtain 0.45 g of solid (cobalt-aluminum layered hydroxide), and the yield is about 81%. Such as image 3 As shown, the material has the structural characteristics of layered hydroxides (LDHs); such as Figure 4 As shown, the material is in flake form.
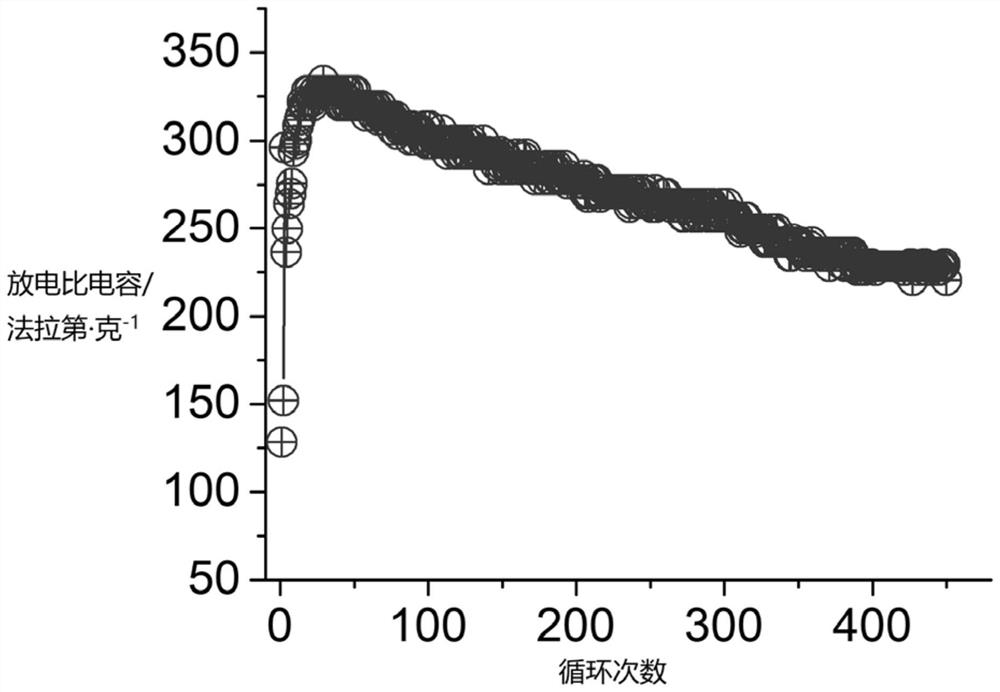
[0044] Take 1.00 g of the solid cobalt-aluminum layered hydroxide prepared above, and add it to 40 mL of potassium hydroxide solution with a concentration of 7 mol / L, then add 0.50 g of polyvinylpyrrolidone and stir evenly; then add 1.00 g of Hexaammine cobalt trichloride, after stirring and dissolving, add 0.50g sodium borohydride, react for 30h, and the reaction temperature is 30°C; after the reaction is ...
Embodiment 3
[0050] Dissolve 11.6g of cobalt nitrate, 15.0g of aluminum nitrate (the molar ratio of cobalt to aluminum is 1:1) and 25.0g of urea (urea) in 300mL of distilled water, raise the temperature to 100°C, keep stirring, and keep warm for 10h to fully precipitate Reaction: After completion of the reaction, filter, wash, and dry to obtain 5.0 g of solid (cobalt-aluminum layered hydroxide), with a yield of about 82%. Such as Figure 5 As shown, the material has the structural characteristics of layered hydroxides (LDHs); such as Figure 6 As shown, the material is flake-like in character.
[0051] Take 1.00 g of the solid cobalt-aluminum layered hydroxide prepared above, and add it to 40 mL of potassium hydroxide solution with a concentration of 7 mol / L, then add 0.50 g of polyvinylpyrrolidone and stir evenly; then add 1.00 g of Hexaammine cobalt trichloride, after stirring and dissolving, add 1.00g sodium borohydride, react for 5h, and the reaction temperature is 30°C; after the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com