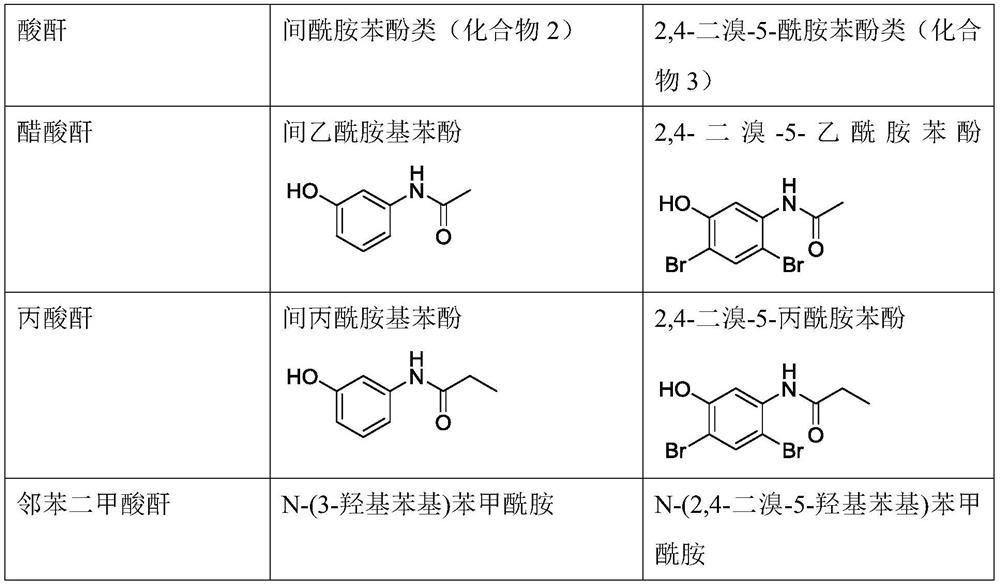
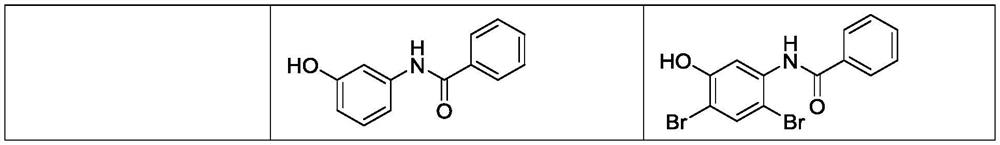
Synthesis method of 5-amino-2, 4-dibromophenol
A technology of dibromophenol and synthesis method, applied in the preparation of amino hydroxy compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve problems such as unfavorable industrial production, difficult control of bromination in photocatalytic reaction, and achieve product yield High, high product purity, environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
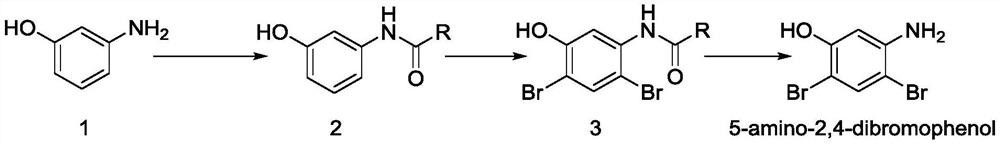
[0041] Example 1, a synthetic method of 5-amino-2,4-dibromobenzol, inter-aminophenol as a raw material, sequentially:
[0042]
[0043] (1) 0.138 mol (about 15.14 g) of amino phenol was added to 100 ml of reaction flask, and 60 ml of methanol was added (i.e., so that amino phenol was dissolved); stirring was lowered to -5 ° C, and the acetic anhydride was added dropwise. 0.166 mol (about 16.99g); temperature control is at -5 ~ 5 ° C, 0.5H dropcard; temperature control at 0 ° C for 2 h, at this time, the sampling point plate determines the reactance of the feedstock;
[0044]The resulting reaction solution was added to 20 ml of ice water, stirred at 0.5 h, 0 ° C to filter, and the resulting filter cake was 11 (2 * 80 mL), dried (at 50 ° C to constant weight), Acetamide-based phenol 18 g (about 0.12 mol), yield is about 87%.
[0045] (2) Dichloromethane and methanol were mixed according to the volume ratio of 5: 2, and the mixture of dichloromethane / methanol was mixed;
[0046] ...
Embodiment 2
[0050] Example 2, a synthetic method of 5-amino-2,4-dibromobenzol, inter-aminophenol as a raw material, sequentially:
[0051] (1) 0.276 mol (approximately 30.28 g) was added to 200 ml of reaction flask (about 30.28 g), and 120 ml of methanol was added, and the mixture was stirred down to -5 ° C, and 0.332 mol (about 34 g) of acetic anhydride was started. -5 ~ 5 ° C, 0.5H dropcard; temperature control at 0 ° C for 2 h, at this time, the sampling point plate determines the reactance of the feedstock;
[0052] The resulting reaction solution was added to ice water (about 40 mL), stirred for 0.5 h, filtration (0 ° C) to give filter cake, ice water twice, dry, greased acetamine phenol 35 g, yield of about 83% .
[0053] (2) Dichloromethane and methanol were mixed according to the volume ratio of 5: 2, and the mixture of dichloromethane / methanol was mixed;
[0054] The metalloacethane-based phenol 12g was administered in a 200 ml reaction bottle, and 120 ml of dichloromethane / metha...
Embodiment 3
[0059] Example 3, a synthetic method of 5-amino-2,4-dibromobenzol, inter-aminophenol as a raw material, specifically the following steps:
[0060] (1) Add a 10 L reaction vessel 13.87 mol (about 1514 g), and then adding methanol 6L to stir well; stirring and cooling to -5 ° C to start dropping acetic anhydride 16.64 mol; temperature control at -5 ~ 5 ° C, 2H is added; the temperature control is at 0 ° C for 2 h, and the sampling point plate determines the reactance of the feedstock;
[0061] The reaction mixture was added to ice water (about 2 L), stirred for 0.5 h, filtration (0 ° C) to give filter cake, ice water twice, dry degree acetamide-based phenol 1.9 kg, yield of about 90.7%.
[0062] (2) Dichloromethane and methanol were mixed according to the volume ratio of 5: 2, and the mixture of dichloromethane / methanol was mixed;
[0063] The sputum-based phenol was put into 10 l (about 600 g) in a 10 L reaction bottle, and the mixture was stirred with dichloromethane / methanol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com