Preparation method of topiroxostat
A technology of topinostat and compounds, applied in the field of drug preparation, can solve the problems of low yield, low purity, unsuitable for industrial production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The invention provides a kind of preparation method of topicastat, comprises the following steps:
[0056] (1) 2-cyanopyridine and hydrazine hydrate are mixed for nucleophilic reaction to obtain a compound having a structure shown in formula II;
[0057]
[0058] (2) Mixing the compound having the structure shown in formula I and the compound having the structure shown in formula II for amidation reaction to obtain the compound having the structure shown in formula III;
[0059]
[0060] The compound having the structure shown in formula I is an acid chloride compound, an ester compound, a mixed anhydride compound or an acyl imidazole compound;
[0061] (3) subjecting the compound having the structure shown in formula III to a ring closure reaction to obtain topicastat.
[0062] The present invention provides and the synthetic route of topicastat such as figure 1 As shown, the following combination figure 1 To elaborate:
[0063] In the present invention, 2-cyan...
Embodiment 1
[0105] The preparation of embodiment 1 2-cyanoisonicotinic acid chloride
[0106] Add 300mL of dichloromethane and 30g of 2-cyano-4-pyridinecarboxylic acid into a 500mL four-neck flask, cool down to 0-5°C in an ice-water bath, add 64.3g of oxalyl chloride, drop 3 drops of DMF, and Insulated and stirred for 3 hours, then slowly raised to room temperature and reacted for 24 hours. After the reaction was completed, concentrated to dryness under reduced pressure to obtain 33.4 g of oily 2-cyanoisonicotinic acid chloride, with a yield of 99.4%.
Embodiment 2
[0107] Example 2 Preparation of methyl 2-cyanoisonicotinate
[0108] Add 20 g of 2-cyano-4-pyridinecarboxylic acid and 19.3 g of thionyl chloride into the three-necked flask, stir at room temperature for 30 minutes, add 40 g of methanol dropwise, stir at room temperature for 3 hours, then raise the temperature to 45°C for 2 hours, after the reaction is completed, Concentrate to dryness under reduced pressure, add 200mL dichloromethane, cool down to -5°C, adjust the pH to 7-8 with sodium carbonate, separate the organic layer, dry the organic layer with anhydrous sodium sulfate and filter, and concentrate the filtrate to dryness under reduced pressure 20.3 g of methyl 2-cyanoisonicotinate was obtained as a white solid, with a yield of 92.7%.
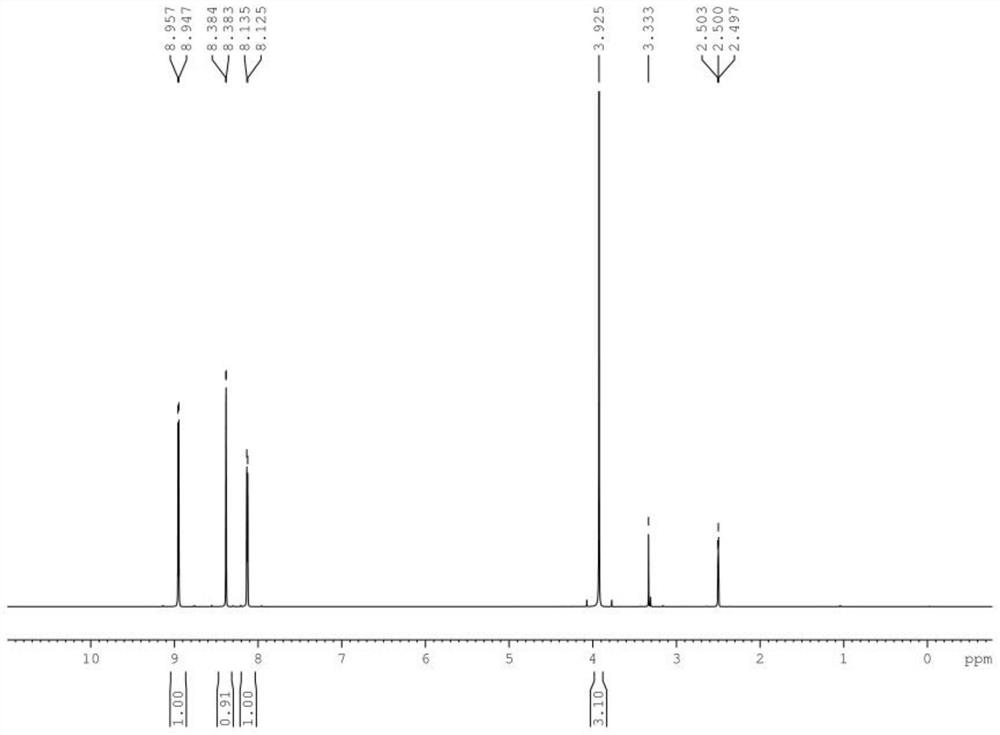
[0109] The NMR spectrum of the product is as figure 1 Therefore, the NMR data are as follows: 1 H-NMR (500MHz, DMSO-d6) δ: 8.96(1H,d), 8.38(1H,d), 8.14(1H,d), 3.93(3H,s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com