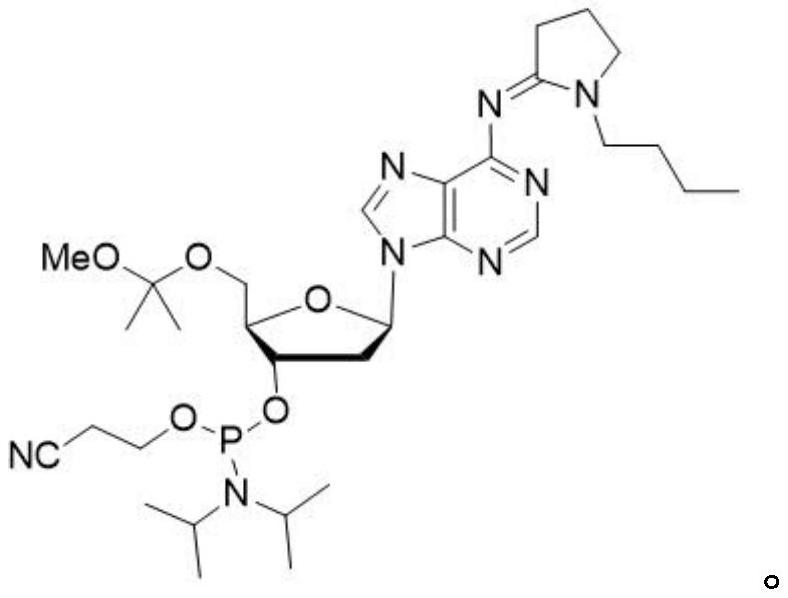
Phosphoramidite nucleoside derivative as well as synthesis method, application and kit thereof
A technology of nucleoside derivatives and synthesis methods, applied in the direction of sugar derivatives, sugar derivatives, sugar derivatives preparation, etc., which can solve the problems of limiting the research and application of oligonucleotide drugs, high preparation costs, and difficulty in purification. Achieve good industrial application value, facilitate mass production, and be easy to purify
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
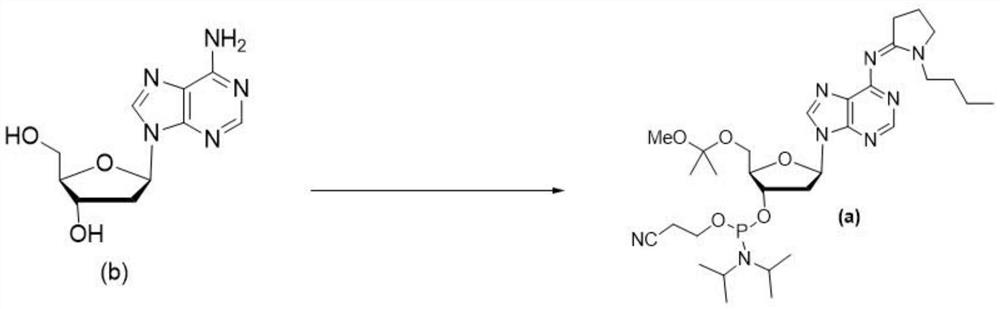
Embodiment 1
[0084] Synthesis of intermediate c:
[0085]
[0086] Steps:
[0087] Dissolve compound b (190g, 0.756mol, 1.0eq.) in pyridine (1.9L), add TMSCl (329g, 3.024mol, 4.00eq.), and react at 25±5°C for 2 hours.
[0088] Then add Bz 2 O (dibenzoyl peroxide, 342g, 1.512mol, 2.0eq.), the reaction solution was heated to 60±5°C and stirred for 8 hours, then added water (400mL) and stirred for 1 hour.
[0089] Subsequently, the reaction solution was directly concentrated to dryness, reconstituted by adding EA (ethyl acetate, 1.9 L), and washed with water (1 L) each time, twice in total. After washing, liquid separation was carried out, and the separated organic phase was dried with anhydrous sodium sulfate and concentrated to dryness at 35±5°C to obtain a crude product. The crude product was suspended and stirred in EtOH / EA (ethanol:ethyl acetate; 1.8L / 0.2L) for 30 minutes, filtered, and dried in vacuo to obtain 200g of compound c, with an HPLC purity of 98.5% and a molar yield of 74%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com