Recombinant chicken interleukin-9 protein and preparation and application of antibody of recombinant chicken interleukin-9 protein
A protein and cell technology, applied in the direction of interleukin, anti-cytokine/lymphokine/interferon immunoglobulin, cytokine/lymphokine/interferon, etc., can solve the problem of unclear molecular weight of chicken IL-9 , to achieve the effect of easy mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1c
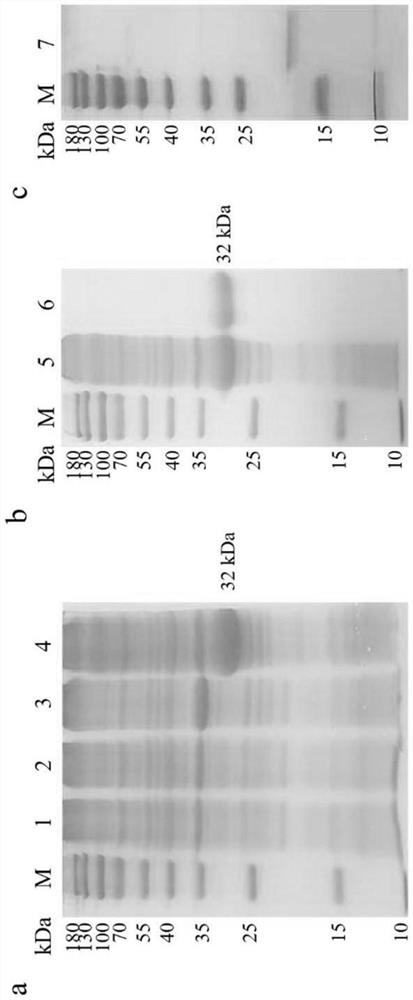
[0033] Embodiment 1 chIL-9 recombinant protein preparation
[0034] 1. ChIL-9 prokaryotic protein expression
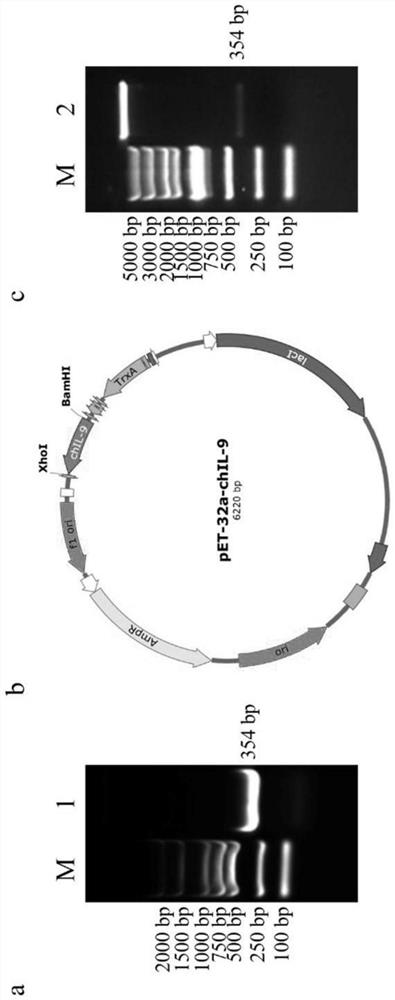
[0035] Because chicken gene codons have low adaptability to Escherichia coli hosts, the cloned chIL-9 nucleotide sequence (as shown in SEQ ID NO.1) without signal peptide was first optimized to be used more frequently in Escherichia coli. a. PCR primers were designed using Primer5.0 software, and restriction sites BamH Ⅰ and Xho Ⅰ and tev protease cleavage sites were introduced for RT-PCR amplification and cloned into the prokaryotic expression vector pET-32a ( figure 1 ), and finally transformed into ROSETTA expression host bacteria.
[0036] PCR primer sequence (shown in SEQ ID NO.5 and 6):
[0037] F:5'-CGGGATCC(BamHI)GAGAACCTGTACTTCCAAGGG(tev)CAGAATTGCCAGGTT-3'
[0038] R: 5'-CCGCTCGAG(XhoI)TCACACGCGGCTTTTAT-3'.
[0039] After the recombinant plasmid containing the target gene was transformed into ROSETTA host bacteria, it was induced by a small amount of IPT...
Embodiment 2c
[0046] The biological function of embodiment 2chIL-9 recombinant protein
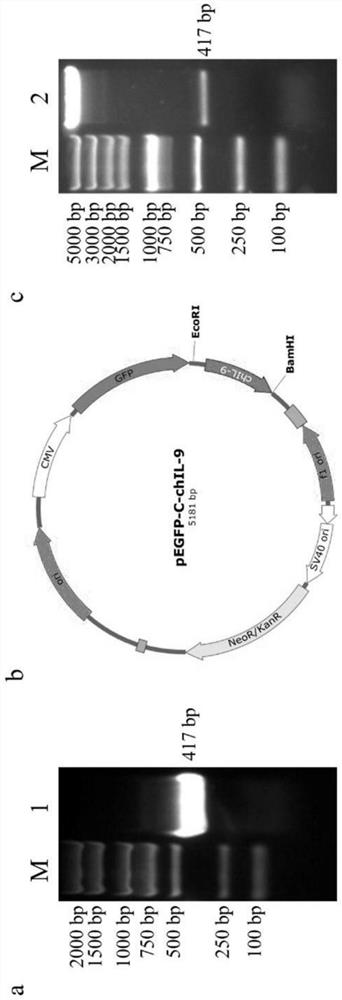
[0047] 1. Activation of chIL-9 recombinant eukaryotic protein on chicken macrophages
[0048] In this experiment, chicken HD11 macrophage cell line and chicken peripheral blood mononuclear cells adhered primary macrophages were selected to detect the function of chIL-9 recombinant protein. The supernatant of pEGFP-C plasmid transfection was added to the control group, the supernatant of pEGFP-C-chIL-9 plasmid transfection was added to the chIL-9 group, and the LPS group was used as a positive control; RNA was extracted after 6 hours of treatment, reverse transcribed into cDNA, and detected by qPCR Changes of key macrophage activating factors IL-1β, IL-6 and iNOS. Figure 5 The results showed that recombinant eukaryotic chIL-9 could activate chicken macrophages to produce inflammatory cytokines.
[0049] 2. The effect of chIL-9 recombinant protein on the proliferation of chicken T cells
[0050] In th...
Embodiment 3c
[0051] Example 3 Preparation of chIL-9 mouse monoclonal antibody
[0052] The main steps of monoclonal antibody preparation include: mouse immunization, cell fusion, screening of hybridoma cells, cell monocloning, antibody identification, ascites preparation and purification.
[0053] 1. Screening of positive hybridoma cells
[0054] 6-week-old female BALB / c mice were selected, and 40 μg of prokaryotically expressed chIL-9 was mixed and emulsified with Freund's adjuvant. Mice were immunized three times by intraperitoneal injection, with an interval of two weeks between each immunization. After 7 days, blood was collected from the tail vein to measure the titer, and the immunization was strengthened. Three days later, the splenocytes of the mice were fused with SP2 / 0 cells under the action of 50% PEG-1500 , and added to feeder cells containing HAT medium, placed at 37 ° C, 5% CO 2 in the cell culture incubator. After 5 days, half of the medium was changed, and after 10 days,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com