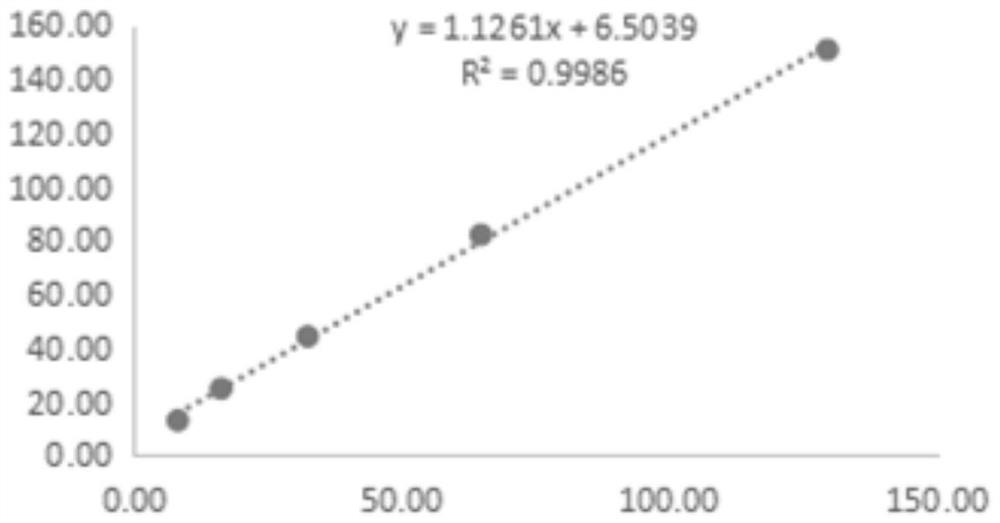
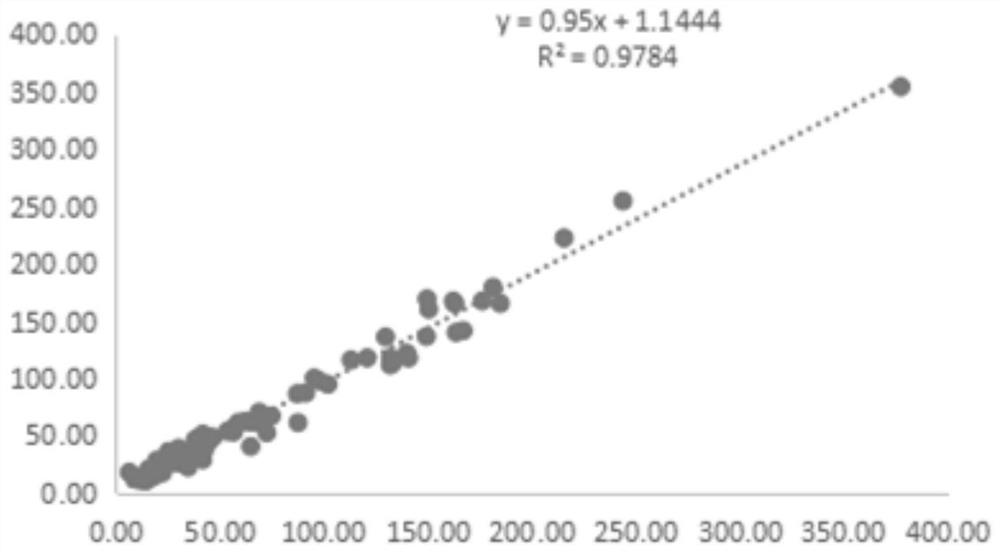
Kit for detecting soluble ST2 protein
A kit and soluble technology, applied in the field of immunomedical detection, can solve problems such as operation errors, and achieve the effects of simple luminescence system, wide linear range and good clinical compliance rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] In this embodiment, the tracer marker is acridinium ester, including:
[0083] Preparation 1: Preparation of primary antibody coated with magnetic beads
[0084] (1) Measure 10mg of magnetic beads (average particle size 3μm, JSR company, solid content 10%), suspend in 1ml 0.1M pH5.5 MES buffer, shake and mix for a short time, use magnet or magnetic stand to absorb for 5-10min Afterwards, discard the supernatant, repeat the above washing step 3 times, add 1ml 0.1M pH5.5 MES buffer, and vortex to mix.
[0085] (2) Add 0.4 mg of ST2 monoclonal antibody (Medix, 100682) at a ratio of magnetic beads:antibody mass ratio of 100:4 to coat in 0.1 M MES buffer, and incubate for 2 hours at room temperature with rotation.
[0086] (3) Add 50 μL of 30 mg / ml 1-(3-dimethylaminopropyl)-3-ethyldiimine hydrochloride (EDC), vortex and incubate at room temperature for 0.5 h.
[0087](4) Add 140 μL 0.15M glycine solution and 70 μL 0.15M ethanolamine solution to the coated antibody, rotate ...
Embodiment 2
[0116] This example provides the screening process of the antibody coating ratio during the preparation of the first antibody.
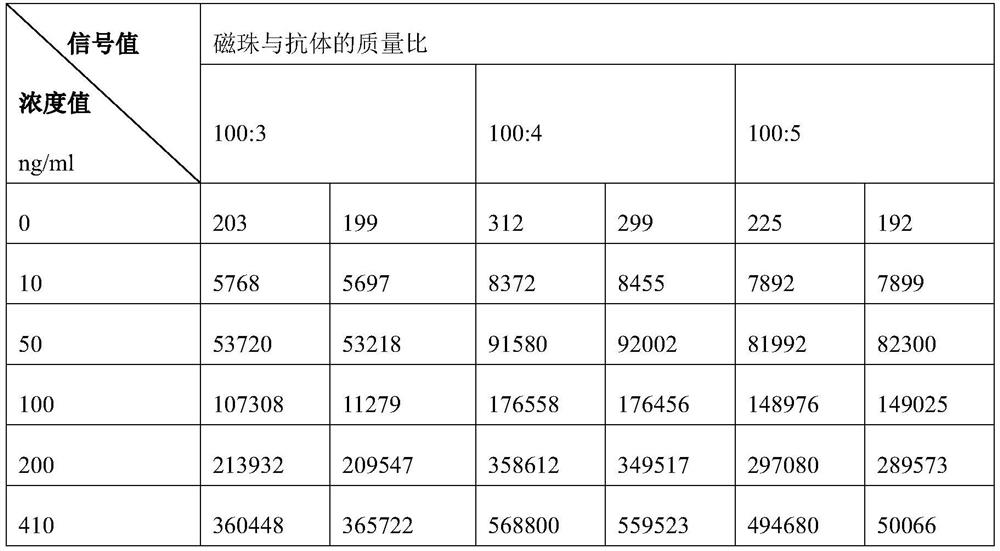
[0117] Compared with the process of Preparation 1 in Example 1, Example 2 changes the mass ratio of the magnetic beads to the antibody in Step (4) of Preparation 1. In Example 2, in step (4) of Preparation 1, 100:3, 100:4, and 100:5 were respectively used as the mass ratio of magnetic beads to antibodies. Combine the three primary antibodies prepared in Example 2 with the secondary antibodies prepared in Example 1 and 2 to obtain three kits, and then cooperate with a fully automatic chemiluminescence analyzer for detection, and each group is repeated twice. Second, the test results are shown in Table 1.
[0118] Table 1 Screening results of the mass ratio of magnetic beads to antibodies during the preparation of the first antibody
[0119]
[0120] The results in Table 1 show that when screening the mass ratio of magnetic beads to antibodies, th...
Embodiment 3
[0122] This example provides the screening process of the antibody and magnetic bead coupling time (ie, coating time) during the preparation of the first antibody.
[0123] Compared with the process of Preparation 1 in Example 1, Example 3 changed the incubation time of step (4) in Preparation 1. In Example 3, in step (4) of Preparation 1, 1 h, 2 h and 3 h were respectively used as the incubation time. Combine the three primary antibodies prepared in Example 3 with the secondary antibodies prepared in Example 1 and 2 respectively to obtain three kits, and then cooperate with a fully automatic chemiluminescence analyzer for detection, and repeat twice for each group. Second, the test results are shown in Table 2.
[0124] Table 2 Screening results of antibody and magnetic bead coupling time
[0125]
[0126]
[0127] The results in Table 2 show that the coupling time of the antibody and the magnetic beads basically reached equilibrium at 2 hours. Therefore, in the prep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Final concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com