Pharmaceutical composition ethosome, gel, hydrogel ointment, patch and preparation method
A technology of alcohol body and composition, applied in the field of pharmaceutical preparation synthesis, can solve the problems of easy relapse administration, inconvenience, unsatisfactory treatment effect and the like, and achieve the effects of good compliance, convenient administration and lasting treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of pharmaceutical composition ethosome:
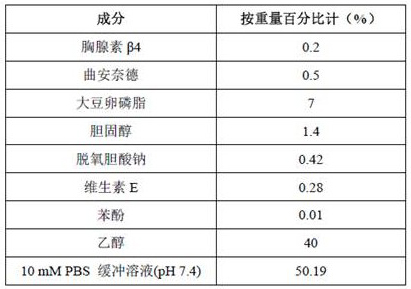
[0038] The composition of ethosomes:
[0039]
[0040] Ethosome preparation method:
[0041] (1) Weigh the amount of soybean lecithin and cholesterol dissolved in ethanol to obtain alcohol phase A;
[0042] (2) Weigh the formula amount of thymosin β4, triamcinolone acetonide, sodium deoxycholate, vitamin E and phenol and dissolve in PBS buffer to obtain aqueous phase B;
[0043] (3) Magnetically stir alcohol phase A and water phase B, and add water phase B to alcohol phase A at a temperature of 33°C; among them, use PBS buffer (phosphate buffered saline, phosphate buffered saline) The total weight of is 100%, and the adding speed of aqueous phase B is 3% / minute;
[0044] (4) Continue magnetic stirring under the same conditions, filter after hydration for 20 minutes, and obtain the ethosome of the pharmaceutical composition;
[0045] (5) Sizing the ethosomes of the obtained pharmaceutical composition by using a...
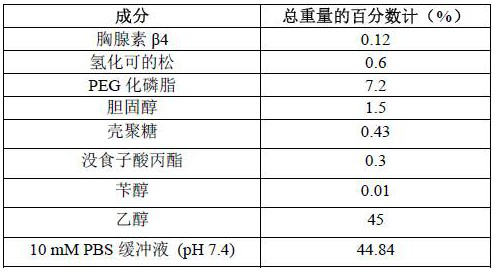
Embodiment 2-8
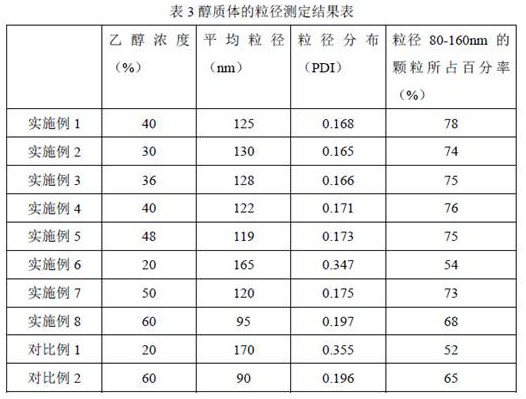
[0051] The preparation method of the ethosome gelling agent of the pharmaceutical composition of Examples 2-8 is the same as that of Example 1, except that the formulation of each component in the ethosome is shown in Table 1 for details.
[0052]
Embodiment 9
[0056] Encapsulation efficiency determination:
[0057] In this example, the ethosome encapsulation efficiency of the pharmaceutical composition obtained in Examples 1-8 was determined by low-temperature ultracentrifugation.
[0058] Precisely pipette 2.0 mL of ethosomes, the pharmaceutical composition obtained in Examples 1-8, into a centrifuge tube, centrifuge at a low temperature of 4°C and 14,000 rpm for 30 min, remove the supernatant, and fill up an equal amount of blank spacer. The supernatant (that is, the prepared supernatant with all components except the pharmaceutical composition removed), centrifuged for 30 minutes under the same conditions, removed and combined the supernatant, HPLC (High Performance Liquid Chromatography) Determination of the amount of the pharmaceutical composition in the supernatant is denoted as M; after the ethosomes of the pharmaceutical composition obtained by centrifugal sedimentation are demulsified (0.2mL methanol demulsification), the a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com