Method for preparing monovalent selective cation exchange membrane through modification
A cation exchange membrane and selective technology, applied in the field of membrane separation, can solve the problems of long reaction time, poor stability of the modified layer, complicated equipment and operation, etc., and achieve easy large-scale production, good membrane selectivity, and simple operation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1) Use Japanese Fuji Type 12 cation exchange membrane as the base membrane, first soak it in a ferric chloride solution with a concentration of 0.01mol / L, take it out after soaking for 0.5 hours, and wash the surface with deionized water to obtain iron-type cations exchange membrane.
[0019] 2) Prepare a Tris-HCl co-deposition buffer solution of dopamine and polyethyleneimine, wherein the concentration of Tris-HCl is 10mmol / L, the concentration of dopamine is 2g / L, and the concentration of polyethyleneimine with a molecular weight of 100000 is 4g / L. Soak the above-mentioned iron-type cation exchange membrane in the prepared coprecipitation buffer solution, place it in a shaker at room temperature for vibration deposition, and carry out surface deposition modification. After 0.5 hours, the membrane was taken out, and the surface was washed with deionized water to obtain a monovalent selective cation exchange membrane.
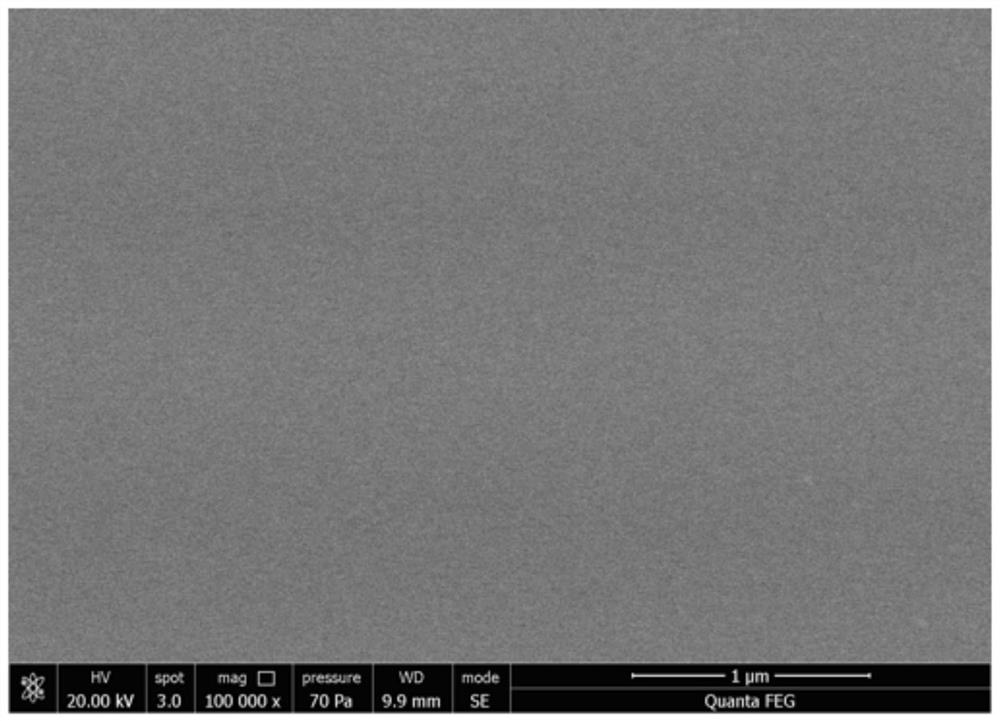
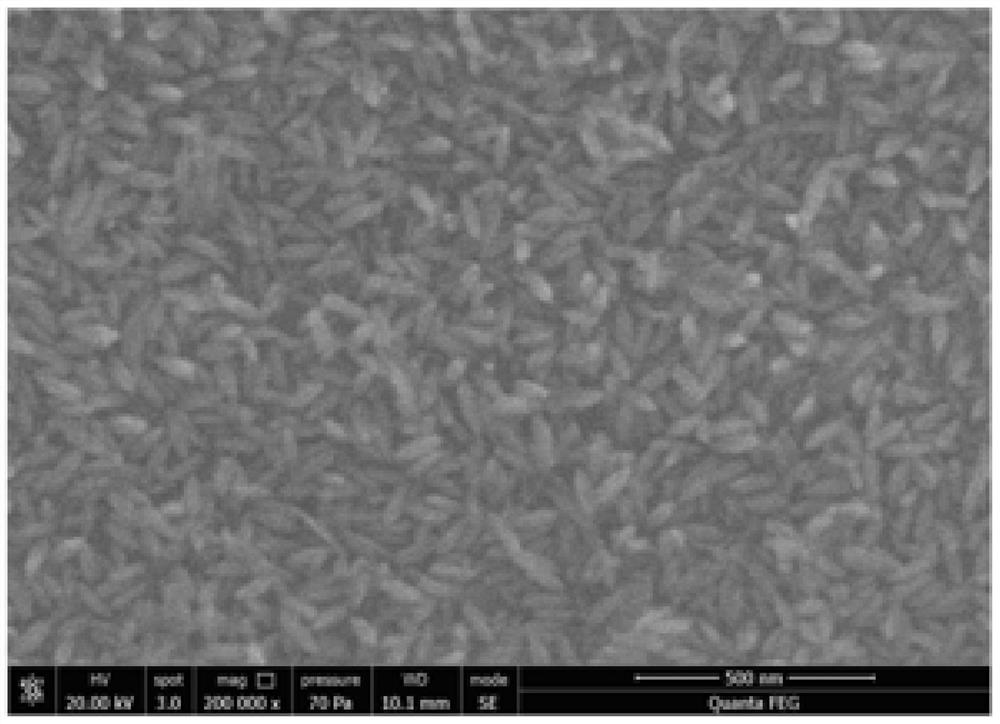
[0020] The scanning electron microscope of the pr...
Embodiment 2
[0023] The ferric chloride solution in the step 1 among the embodiment 1 is changed into the ferric nitrate solution of 1mol / L, and the oscillation deposition time in the shaking table is changed into 6 hours in the step 2, all the other materials used are consistent with the operating method, and obtained The monovalent selective cation exchange membrane described above.
[0024] After testing, the performance of the ion-exchange membrane prepared is as follows: the membrane resistance is increased by 6.0Ω·cm of the base membrane 2 Increased to 7.85Ω·cm 2 . NaCl and MgCl with a concentration of 0.1mol / L 2 The solution is a raw material solution, and a selectivity test is carried out in an electrodialysis device, and the selectivity coefficient of the prepared monovalent selective ion exchange membrane is 4.2.
Embodiment 3
[0026] The ferric chloride solution in the step 1 among the embodiment 1 is changed to the ferric sulfate solution of 0.1mol / L, the dopamine concentration in the step 2 co-deposition buffer is 10g / L, and using molecular weight is the polyethyleneimine of 10000, its The concentration is 10g / L, and the other materials used are consistent with the operating method to prepare the monovalent selective cation exchange membrane.
[0027] After testing, the performance of the ion-exchange membrane prepared is as follows: the membrane resistance is increased by 6.0Ω·cm of the base membrane 2 Increased to 7.42Ω·cm 2 . NaCl and MgCl with a concentration of 0.1mol / L 2 The solution is a raw material solution, and a selectivity test is carried out in an electrodialysis device, and the selectivity coefficient of the prepared monovalent selective ion exchange membrane is 4.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com