Chlamydia pneumoniae antigen detection plate and detection box
A technology for detection of Chlamydia pneumoniae and antigens, which is applied to measuring devices, instruments, fluorescence/phosphorescence, etc., can solve the problems of lack of fast, simple and reliable methods, cumbersome operations, and long time required to achieve uniformity and stability performance, improve repeatability, and improve stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
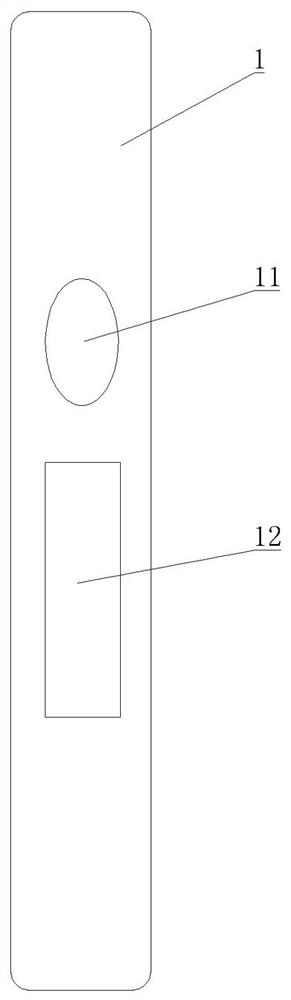
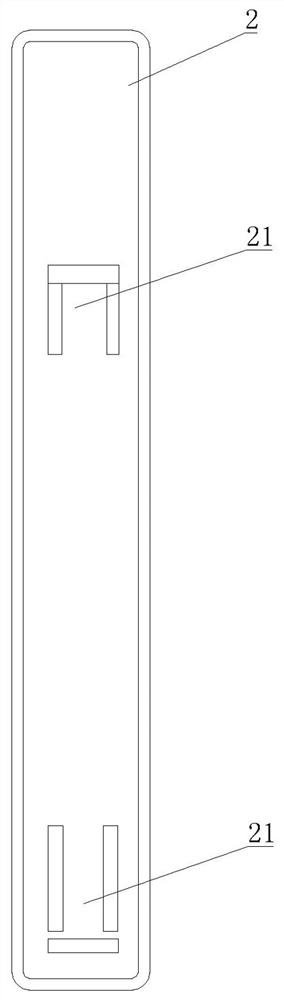
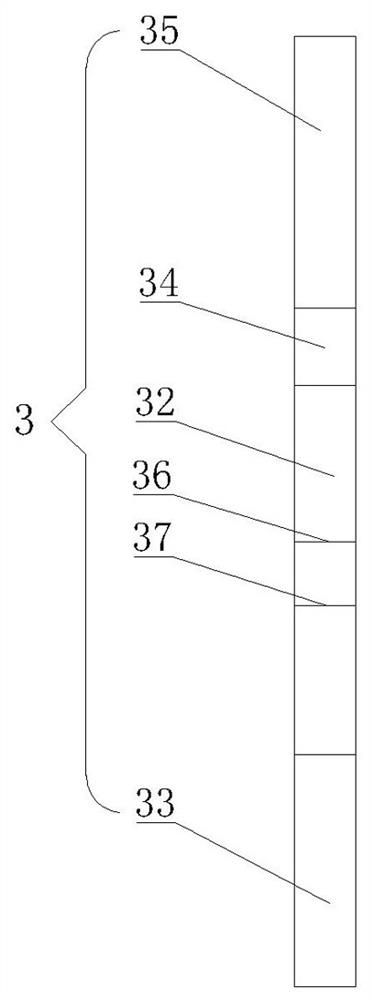
[0035] Embodiment one: as attached figure 1 - attached Figure 4As shown, a Chlamydia pneumoniae antigen detection plate comprises a sealed upper cover 1 and a lower cover 2, the upper cover 1 is provided with a sampling hole 11 and an observation window 12, and the lower cover 2 is provided with a card Slot 21, a test paper strip 3 is placed in the card slot 21, and the test paper strip 3 includes a PVC lath 31, and the PVC lath 31 is provided with a nitrocellulose membrane 32, and the nitrocellulose membrane 32 The position corresponds to the position of the observation window 12, the end of the nitrocellulose membrane 32 away from the sample injection hole 11 is provided with an absorbent paper 33, and the other end of the nitrocellulose membrane 32 is provided with a bonding pad 34 , and the binding pad 34 is completely covered by the upper cover 1 , the free end of the binding pad 34 is provided with a sample pad 35 , and the position of the sample loading hole 11 corres...
Embodiment 2
[0046] Embodiment 2: A Chlamydia pneumoniae antigen detection kit, including the detection plate described in Embodiment 1, and also includes an extraction tube, a disposable sampling swab, and a sample detection solution.
[0047] The sample detection solution is composed of 20mmol / L, Tris-HCL buffer solution with pH of 8, S9 with a mass fraction of 2.5%, Nacl with a mass fraction of 0.9%, Tween with a volume fraction of 0.01%, and Tween with a mass fraction of 0.1%. casein.
[0048] The detection of this kit adopts the immunofluorescence double-antibody sandwich method combined with the amplification system. Its basic principle is the combination of immunochromatography detection technology and amplification system labeling technology. Biotin-labeled Chlamydia pneumoniae antibody and fluorescently-labeled Chlamydia pneumoniae antibody, nitrocellulose membrane 32 coated with avidin and goat anti-mouse quality control line, when the sample contains Chlamydia pneumoniae antigen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com