Broad-spectrum humanized anti-novel coronavirus monoclonal antibody and application thereof
A monoclonal antibody and coronavirus technology, applied in the field of peptides, can solve the problems of failure, Omicron neutralization ability decline, etc., and achieve the effect of good stability and significant broad-spectrum neutralization ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
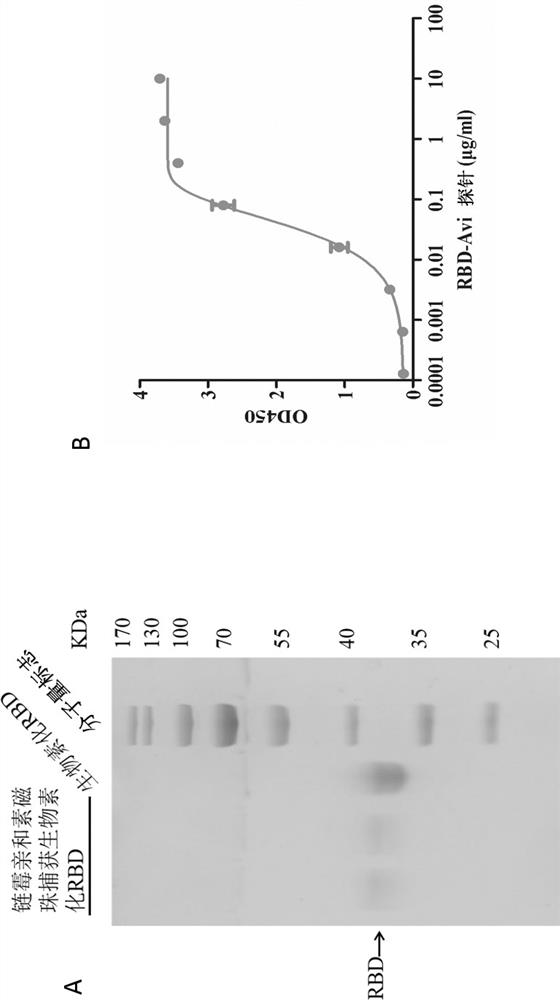
[0042] Example 1: Synthesis, expression, biotinylation and staining of the novel coronavirus RBD probe
[0043] 1.1 According to the data published by Genbank (NC_045512), synthetically carry 6×His-Avi (His-His-His-His-His-His-Glu-Lys-Asn-Glu-Gln-Glu-Leu-Leu-Glu-Leu- Asp-Lys-Trp-Ala-Ser-Leu-Trp-Asn-Trp-Phe-Asp-Ile-Thr-Asn-Trp-Leu-Trp-Tyr-ILe-Lys-Lys-Lys) tagged RBD full-length gene sequence.
[0044] 1.2 Reconnected into the eukaryotic expression vector pDRVI1.0 (constructed and preserved by the inventor) after EcoRI and EcoRV double digestion, and the clone was selected and sequenced without error.
[0045] 1.3 The two probe plasmids were respectively transfected into 293F cells for expression. After 5-6 days, the culture medium was centrifuged to collect the cell supernatant, and the antigenic protein was purified through a nickel column.
[0046] 1.4 Use the BirA 500 Biotin Protein Ligase Kit (BirA500, Avidity) to biotinylate the probe protein:
[0047] Dissolve 1 mg mol...
Embodiment 2
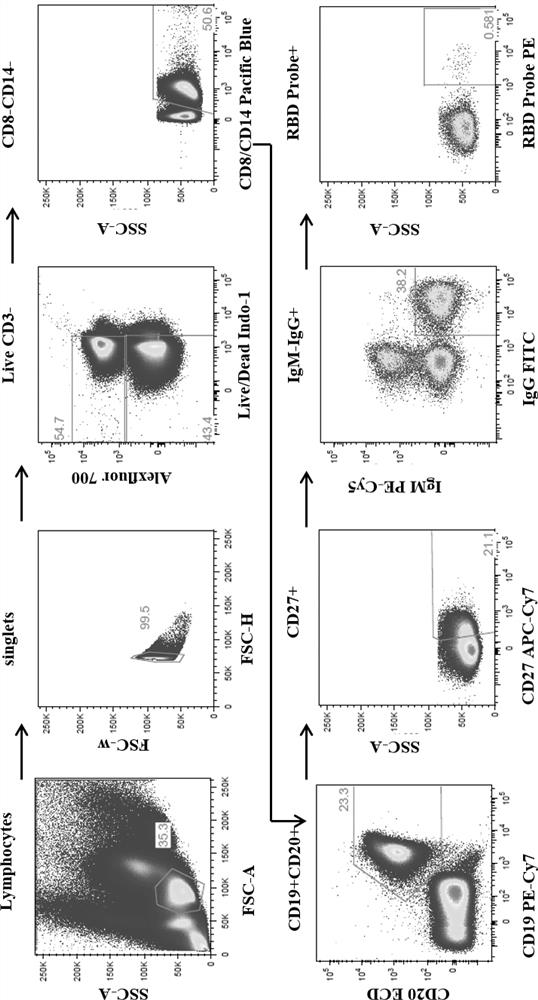
[0053] Embodiment 2: screening and identification of anti-SARS-COV-2 humanized monoclonal antibody
[0054] 2.1 Prepare cell lysate: 20 μl cell lysate per well, including 0.5 μl RNase removal, 5 μl 5×FirstStrand buffer, 1.25 μL 0.1M DTT, 0.0625 μl Igepal, 13.25 μl water, cover with sealing film, 4°C Store in the refrigerator for later use.
[0055] 2.2 Sample preparation:
[0056] (1) Resuscitation of PBMCs cells from recovered patients after COVID-19 infection: Take out the frozen cell tube from liquid nitrogen, put it in a 37°C water bath quickly, take it out when it melts until there is an ice core, open it in a biological safety cabinet, and slowly Add R10+ Benzonase medium dropwise (5 mL R10+ Benzonase medium is used for 1 branch of cells). Centrifuge at 1500 rpm for 10 minutes, discard the supernatant, suspend the cells with the residual solution, add 10 mL R10, mix well, take 50 μl for cell counting, and centrifuge at 1500 rpm for 10 minutes; adjust the cell concentra...
Embodiment 3
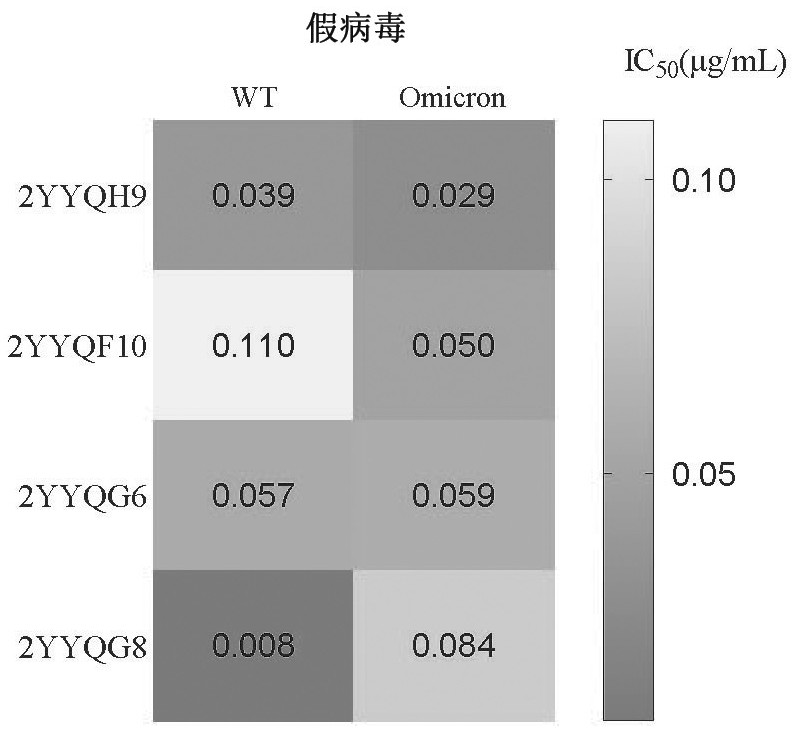
[0106] Embodiment 3 Antibody is to the neutralizing activity determination of SARS-COV-2 pseudovirus
[0107] 3.1 Packaging of pseudovirus: The full-length S protein gene of artificially synthesized SARS-CoV-2 (GenBank: MN908947) was inserted into the pcDNA3.1 expression plasmid to construct pcDNA-SARS-CoV2-S. The pseudovirus mutation site with mutation is introduced on the S gene as shown in Table 11.
[0108] Table 11. Pseudovirus mutation sites
[0109] .
[0110] will be 3×10 6 (3 million) 293T / 17 cells were seeded in T75 cell culture flasks, 5% CO 2 Incubate at 37°C for 20-24 hours. Use Fugene 6 Transfection Reagent (Promega, Cat#E2691) for transfection: 30ug plasmid pcDNA-SARS-CoV2-S was transfected into 293T cells in a T75 culture flask, and 1.05×10 6 The G*ΔG-VSV virus with TCID50 infected 293T, and the culture medium was changed after 8 hours. After 24 hours of transfection, the culture supernatant was collected and filtered to obtain the pseudovirus of the S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com