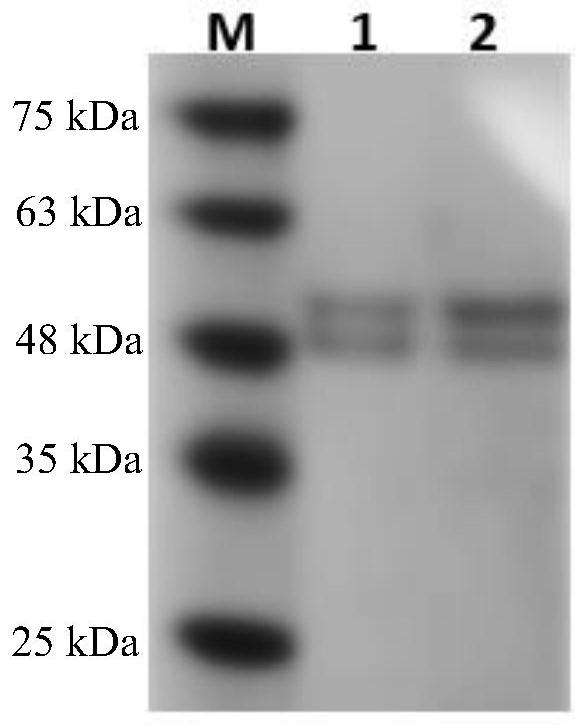
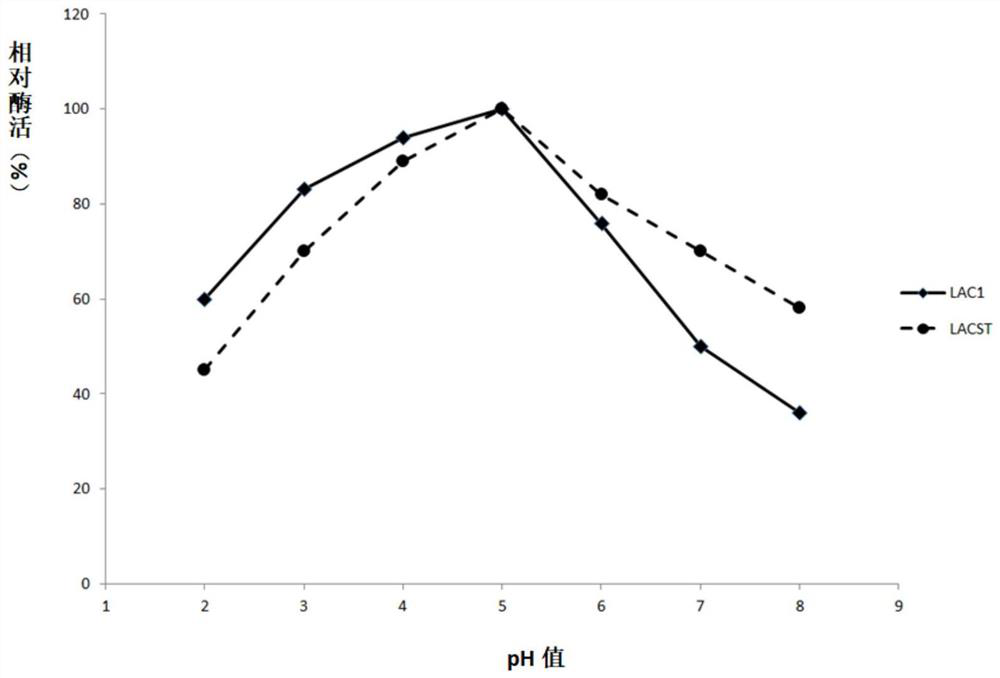
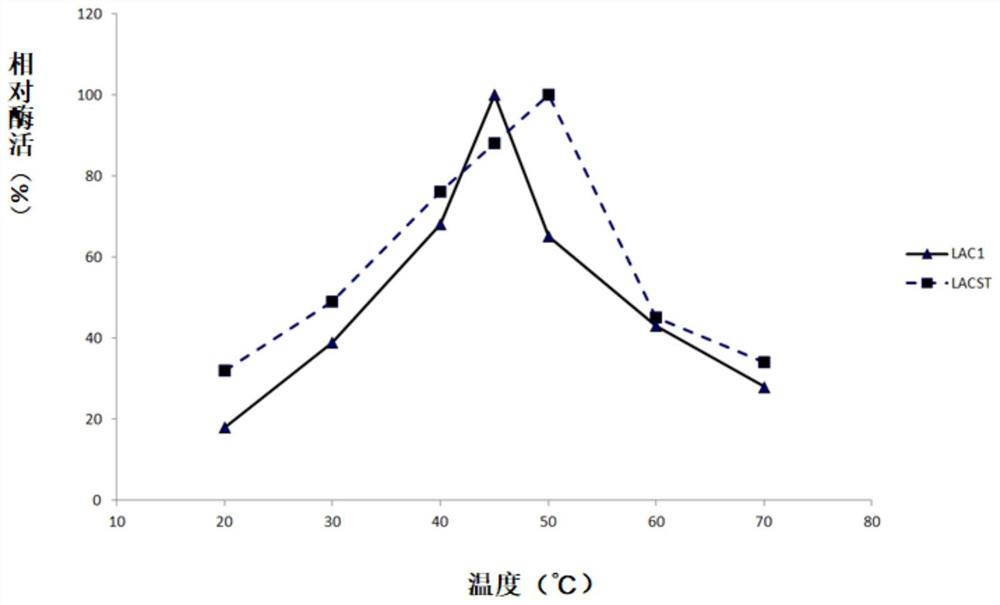
Fungus laccase as well as preparation method and application thereof
A fungal laccase and bacterial strain technology, applied in the biological field, can solve the problems of unstable production and low laccase activity, and achieve the effects of reduced production costs, simple preparation methods, and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1, Extraction of Inonotus roughae XJ-11 total RNA and cloning of laccase gene
[0042] Inoculate PDA culture medium (purchased from Beijing Ai Pu Rui Sheng Technology Co., Ltd.) by inoculating the laboratory-preserved Inonotus pilosa (No.: XJ-11, isolated and preserved by Hunan Lierkang Biological Co., Ltd.) , and cultivated at 30°C for 72h. Centrifuge at 12,000 rpm for 10 minutes to collect the bacteria, wash with 10 mL of sterilized water for 2 to 3 times, place the bacteria in a mortar, add liquid nitrogen to freeze, grind and break the bacteria. Total RNA was extracted using a fungal total RNA extraction kit (product of Omega Company).
[0043] Reverse Transcription:
[0044] 5 μL (about 3 μg) of total RNA and 1.0 μL (1.0 μg) of Oligo dT18 were mixed, denatured at 70°C for 5 minutes, and immediately inserted on ice for 2 minutes. Add 10 U of reverse transcriptase AMV, 50 U of RNsin, 3 μL of 0.1 mol / L DTT, 1 μL of 10 mM dNTPs, and react at 42° C. for 1.5 h...
Embodiment 2
[0054] Embodiment 2, construction of laccase gene lac1 expression engineering strain
[0055] Use DNAMAN software to the gene lac1 sequence (SEQ ID NO.5) that embodiment 1 obtains, carry out restriction site analysis, design primer F-e (SEQ ID NO.7): 5'-CG GAATTC ATGGCCAGGTTCCAATCTCT-3' (wherein, the underline is the ECORI site) and R-x (SEQ ID NO.8): 5'-GC TCTAGA TTACTGGTCGTCAGGCGAG-3' (wherein, the underline is the XbaI site). Using the reverse transcription PCR product (cDNA) obtained in Example 1 as a template, PCR amplification was performed.
[0056] The components of the PCR system are as follows: 1 μL of PCR product, 1 μL of Phusion DNA polymerase, 1 μL of primers F-e and R-x (both at a concentration of 10 μM), 1 μL of 10 mM dNTP, 10 μL of 5× reaction buffer, ddH 2 O 35 μL, total volume 50 μL.
[0057] The PCR amplification conditions were as follows: pre-denaturation at 94°C for 3 minutes, followed by 30 cycles of 94°C for 30s, 58°C for 30s, and 72°C for 2min, an...
Embodiment 3
[0061] Embodiment 3, construction of lacST recombinant plasmid of laccase mutant gene
[0062] The key amino acid sites that may affect the activity were predicted by laccase protein advanced structure simulation, and then site-directed mutagenesis was performed.
[0063] According to the measured sequence, the mutation primer sequence was designed, and two mutation sites were introduced. Mutation primers were designed: M1 (SEQ ID NO.9): 5'-ACACGCTCAGCGCCGATTTG-3' and M2 (SEQ ID NO.10): 5'-CCGGCGGTGAAGAGGTCC-3'.
[0064] Use primer M1 / R-x to amplify a 950bp fragment, and use primer M2 / F-e to amplify a 1100bp fragment to obtain a fragment with mutation sites at both ends, and then put them together for overlapping PCR extension to obtain a full-length mutation Body gene, the specific operation is as follows:
[0065] PCR amplification: use primers M1 and R-x and another pair of primers M2 and F-e for PCR amplification respectively, and introduce an amino acid mutation site in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com