Large-scale synthesis method of natural alpha-glucosidase inhibitor Penasullate A
A technology of glucosidase and synthesis method, applied in the directions of organic chemistry method, organic chemistry, drug combination, etc., can solve the problem that PenasulfateA cannot be synthesized on a large scale, and achieve the effect of shortening steps and time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
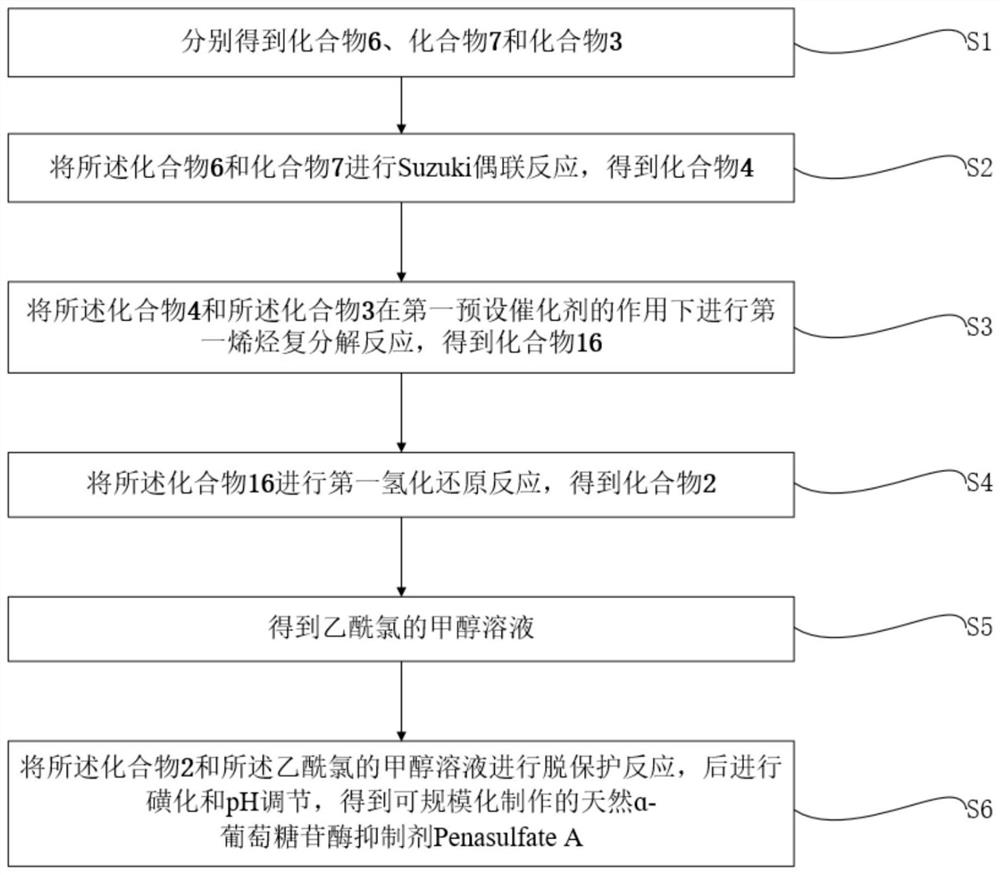
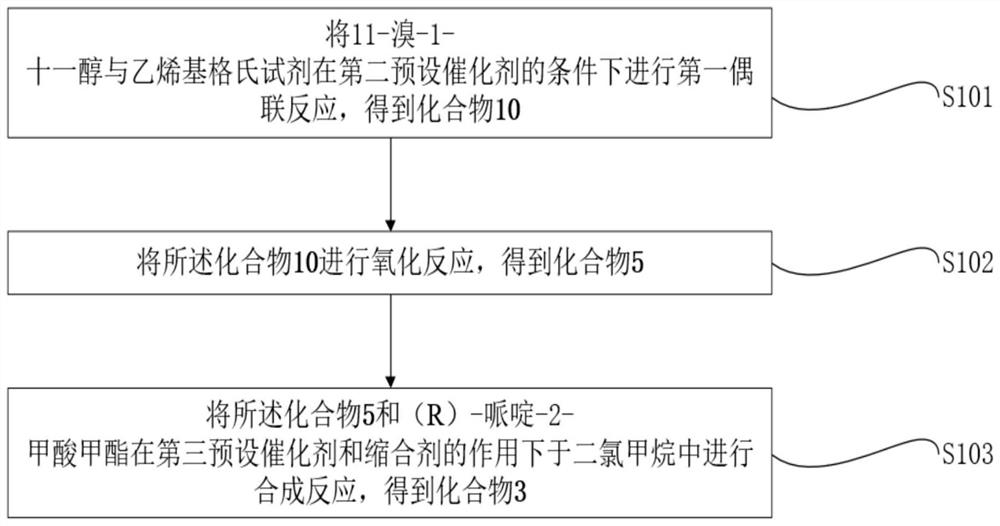
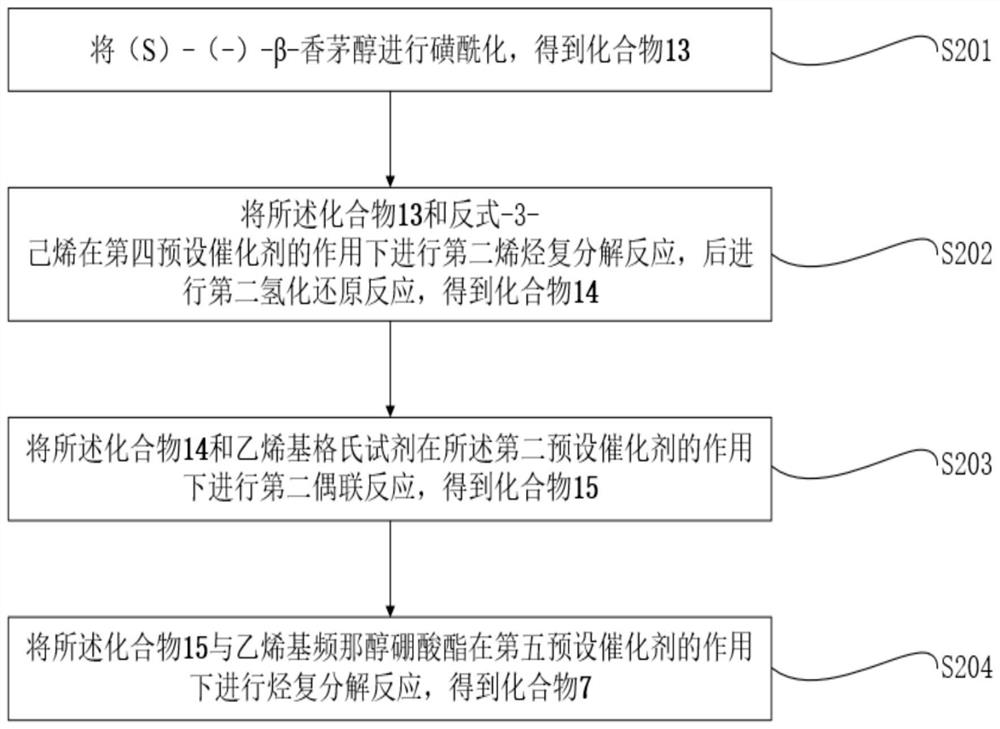
[0066] In one embodiment of this application, as figure 1 , Figure 4 and Figure 5 Shown, provide a kind of large-scale synthetic method of natural α-glucosidase inhibitor Penasulfate A, described method comprises:
[0067] S1. Obtain compound 6, compound 7 and compound 3 respectively;
[0068] S2. The compound 6 and compound 7 were subjected to Suzuki coupling reaction to obtain compound 4;
[0069] S3. performing the first olefin metathesis reaction on the compound 4 and the compound 3 under the action of the first predetermined catalyst to obtain the compound 16;
[0070] S4. Carrying out the first hydrogenation reduction reaction of the compound 16 to obtain the compound 2;
[0071] S5. obtain the methanol solution of acetyl chloride;
[0072] S6. Deprotecting the compound 2 and the methanol solution of acetyl chloride, followed by sulfonation and pH adjustment, to obtain Penasulfate A, a natural α-glucosidase inhibitor that can be produced on a large scale;
[0073...
Embodiment 1
[0120] Such as figure 1 , Figure 4 and Figure 5 Shown, a kind of large-scale synthetic method of natural α-glucosidase inhibitor Penasulfate A, described method comprises:
[0121] S1. Obtain compound 6, compound 7 and compound 3 respectively;
[0122] S2. The compound 6 and compound 7 were subjected to Suzuki coupling reaction to obtain compound 4;
[0123] S3. performing the first olefin metathesis reaction on the compound 4 and the compound 3 under the action of the first predetermined catalyst to obtain the compound 16;
[0124] S4. Carrying out the first hydrogenation reduction reaction of the compound 16 to obtain the compound 2;
[0125] S5. obtain the methanol solution of acetyl chloride;
[0126] S6. Deprotecting the compound 2 and the methanol solution of acetyl chloride, followed by sulfonation and pH adjustment, to obtain Penasulfate A, a natural α-glucosidase inhibitor that can be produced on a large scale;
[0127] Wherein, the general structural formula of...
Embodiment 2
[0165] Compare embodiment 2 and embodiment 1, the difference of embodiment 2 and embodiment 1 is:
[0166] The used oxidizing agent of oxidation reaction comprises sodium chlorite, tetramethyl piperidine oxide and sodium hypochlorite, and the equivalent of sodium chlorite is 2 times of compound 10, and the equivalent of tetramethyl piperidine oxide is 0.05 times of compound 10, and sodium hypochlorite The equivalent of is 0.02 times that of compound 10.
[0167] The reaction temperature of the Suzuki coupling reaction was 25 °C.
[0168] The first preset catalyst is the Hoveyda-Grubbs II catalyst.
[0169] The reaction temperature of the first olefin metathesis reaction is 45° C., and the solvent used in the first olefin metathesis reaction is 1,2-dichloroethane or toluene.
[0170] The reaction time of the first hydrogenation reduction reaction is 0.5 h; the mass fraction of the Pd / C catalyst is 5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com