Magnesium hydride-in-situ generated metal borohydride hydrolysis hydrogen production material and preparation method thereof
A metal borohydride, in-situ generation technology, applied in the directions of alkali metal/alkaline earth metal/beryllium/magnesium hydride, borane/diborane hydride, chemical instruments and methods, etc. Regeneration, high price of calcium hydride, difficult separation and regeneration of hydrolyzed products, etc., to avoid significant decline, increase specific surface area, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
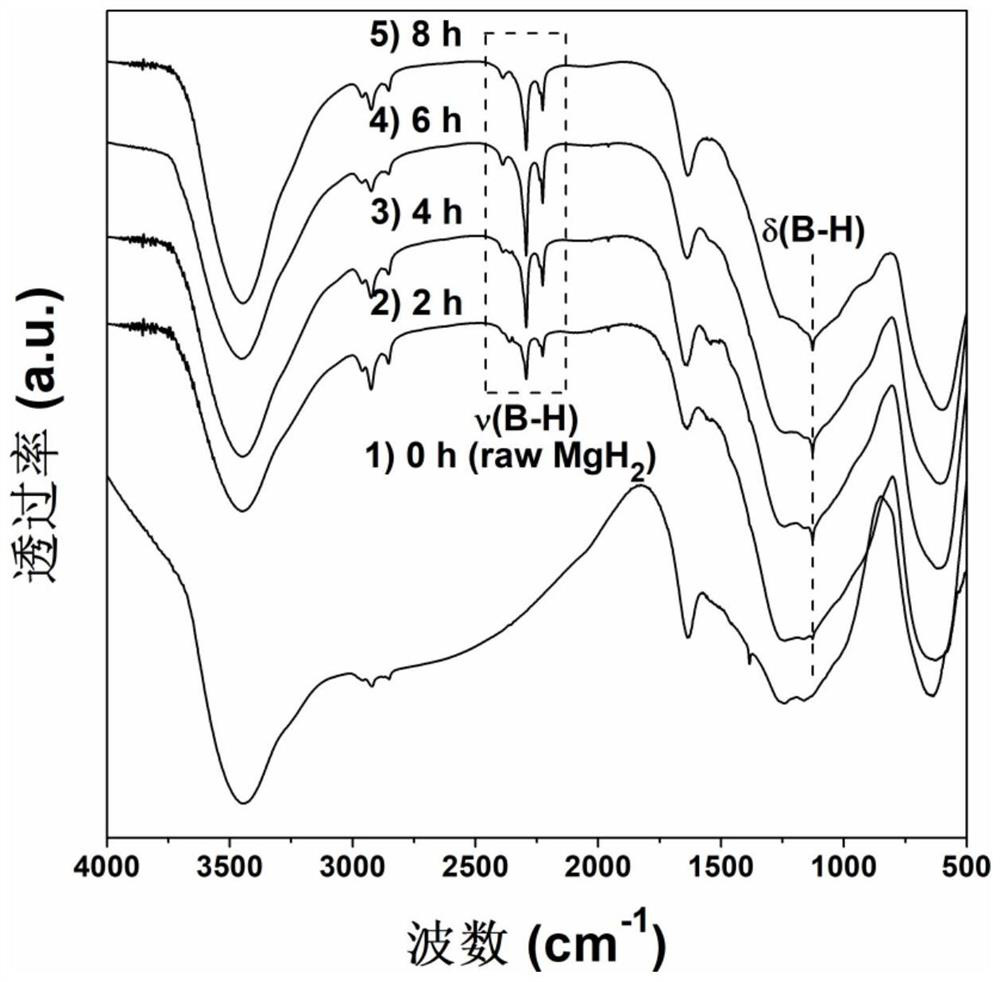
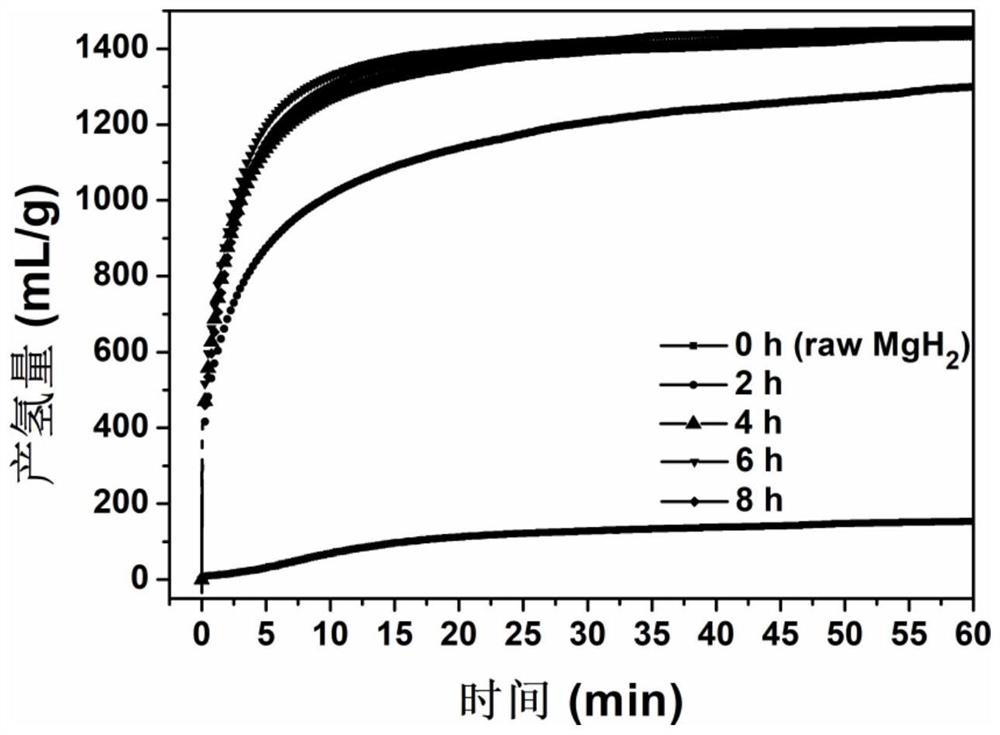
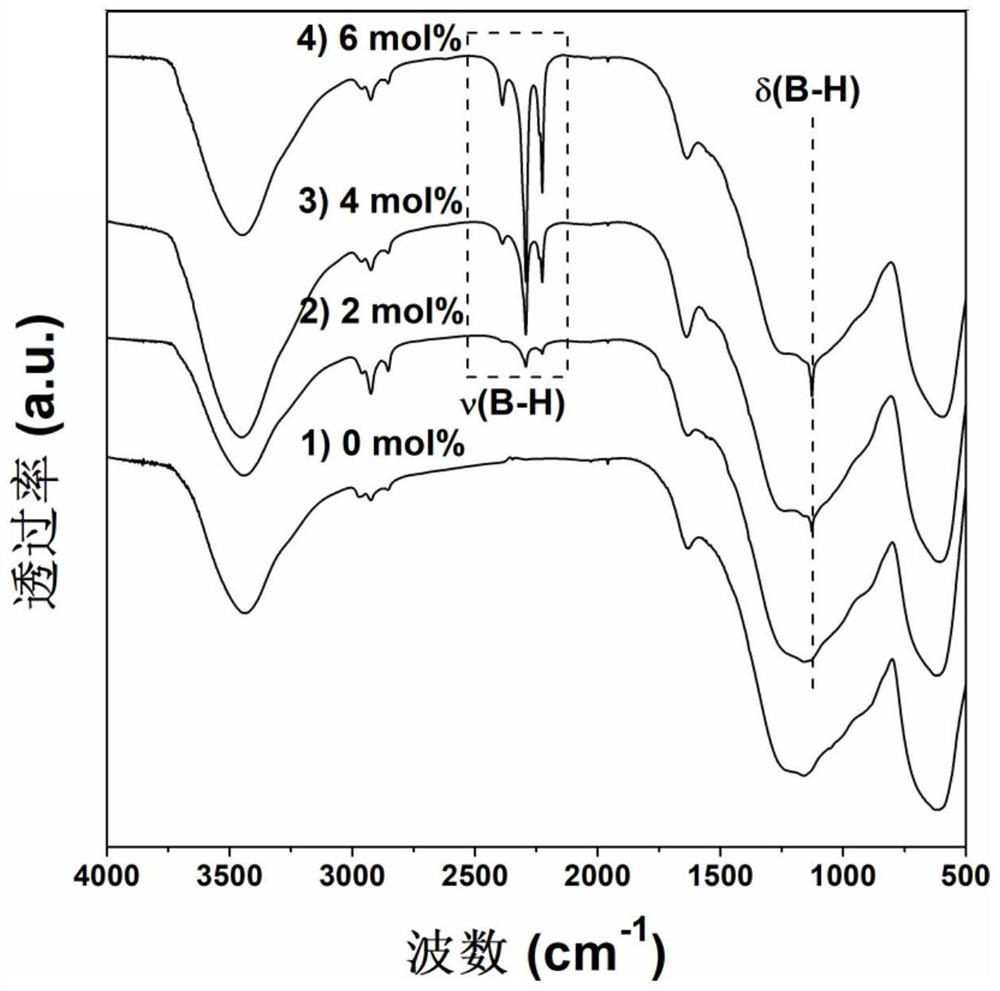
[0043] In a glove box of 0.1MPa argon atmosphere, take magnesium hydride and 4mol% sodium metaborate, mix them and put them into a ball mill jar and place the ball mill jar in a vibration ball mill (QM-3C) with a ball-to-material ratio of 50 : 1, ball milling speed 1000r / min, ball milling directly under the argon atmosphere for 2h, to obtain the ball milling product. figure 1 Middle curve 2) is the FTIR spectrogram of this ball mill product, 2200-2425cm in the curve -1 and 1127cm -1 corresponding to the B-H bond stretching vibration in sodium borohydride ( figure 1 ν) and rocking vibration ( figure 1 The δ) absorption peak in , proves that sodium borohydride was successfully generated in situ. The prepared magnesium hydride-sodium borohydride hydrolysis hydrogen production material was hydrolyzed in pure water at 25°C to release hydrogen, and the hydrolysis performance was significantly improved compared with unmilled magnesium hydride, and 1298.9mL·g was released within 1h...
Embodiment 2
[0045] In a glove box of 0.1MPa argon atmosphere, take magnesium hydride and 4mol% sodium metaborate, mix them and put them into a ball mill jar and place the ball mill jar in a vibration ball mill (QM-3C) with a ball-to-material ratio of 50 : 1, ball milling speed 1000r / min, ball milling 4h directly under this argon atmosphere, obtains ball milling product. figure 1 Middle curve 3) is the FTIR spectrogram of this ball mill product, 2200-2425cm in the curve -1 and 1127cm -1 corresponding to the B-H bond stretching vibration in sodium borohydride ( figure 1 ν) and rocking vibration ( figure 1 The δ) absorption peak in , proves that sodium borohydride was successfully generated in situ. The prepared magnesium hydride-sodium borohydride hydrolysis hydrogen production material was hydrolyzed in pure water at 25°C to release hydrogen, and the hydrolysis performance was significantly improved compared with unmilled magnesium hydride, and the hydrolysis rate was extremely fast. Th...
Embodiment 3
[0047] In a glove box of 0.1MPa argon atmosphere, take magnesium hydride and 4mol% sodium metaborate, mix them and put them into a ball mill jar and place the ball mill jar in a vibration ball mill (QM-3C) with a ball-to-material ratio of 50 : 1, ball milling speed 1000r / min, ball milling directly under the argon atmosphere for 6h, to obtain a ball milling product. figure 1 Middle curve 4) is the FTIR spectrogram of this ball mill product, in the curve 2200-2425cm -1 and 1127cm -1 corresponding to the B-H bond stretching vibration in sodium borohydride ( figure 1 ν) and rocking vibration ( figure 1 The δ) absorption peak in , proves that sodium borohydride was successfully generated in situ. The prepared magnesium hydride-sodium borohydride hydrolysis hydrogen production material was hydrolyzed in pure water at 25°C to release hydrogen, and the hydrolysis performance was significantly improved compared with that of unmilled magnesium hydride, and the average hydrolysis rate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com