Preparation method of cyclopropane derivative
A technology for derivatives and cyclopropane, applied in the field of preparation of cyclopropane derivatives, can solve the problems of difficulty in industrial production of cyclopropane derivatives, extremely high requirements for synthesis equipment and process control, harsh operating conditions, etc., so as to reduce the reaction time. Requirements for equipment and process control, avoiding harsh requirements, and the effect of less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
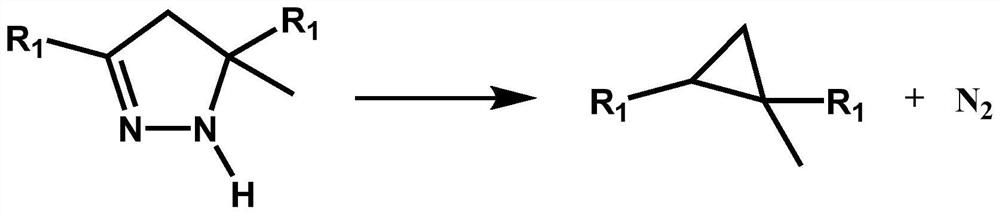
[0065] In this example, 3,5-methyl-5-methylpyrazoline is used to prepare 1,2-methyl-2-methylcyclopropane, and the reaction formula is as follows figure 1 shown, where R 1 For methyl. This embodiment specifically includes the following steps:
[0066] 1) Weigh 112g of 3,5-methyl-5-methylpyrazoline, add it to a 500mL flask, stir it at a stirring speed of 200rpm at room temperature, and add 12g of hydrogen hydroxide Potassium; stop stirring after fully stirring for 2 hours, let the liquid stand for 50 minutes, use liquid separation to separate the lower aqueous phase, and collect a total of 110 g of the upper liquid, which is a dry pyrazoline compound;
[0067] 2) Under normal temperature conditions, transfer the dry pyrazoline compound obtained in step 1) to the reactor, stir at a stirring speed of 300rpm, and add 20g of potassium hydroxide to the reactor; liquid;
[0068] 3) Keep stirring the reaction solution continuously, heat the reaction solution to 160°C, keep it warm,...
Embodiment 2
[0074] In this example, 3,5-cyclopropyl-5-methylpyrazoline is used to prepare 1,2-cyclopropyl-2-methylcyclopropane, and the reaction formula is as follows figure 1 shown, where R 1 For cyclopropyl. This embodiment specifically includes the following steps:
[0075] 1) Weigh 164g of 3,5-cyclopropyl-5-methylpyrazoline, add it to a 500mL flask, stir it at a stirring speed of 200rpm at room temperature, and add 16.4g Potassium hydroxide; stop stirring after fully stirring for 2 hours, let the liquid stand for 50 minutes, use liquid separation to separate the lower aqueous phase, and collect a total of 162 g of the upper liquid, which is a dry pyrazoline compound;
[0076] 2) Under normal temperature conditions, transfer the dried pyrazoline compound obtained in step 1) to the reactor, stir at a stirring speed of 300rpm, and add 32.4g of potassium hydroxide to the reactor; The reaction solution;
[0077] 3) Keep stirring the reaction solution continuously, heat the reaction sol...
Embodiment 3
[0083] In this example, 3,5-methyl-5-methylpyrazoline is used to prepare 1,2-methyl-2-methylcyclopropane, and the reaction formula is as follows figure 1 shown, where R 1 For methyl. This embodiment specifically includes the following steps:
[0084] 1) Weigh 112g of 3,5-methyl-5-methylpyrazoline, add it to a 500mL flask, stir it at a stirring speed of 400rpm at room temperature, and add 12g of hydroxide Potassium; stop stirring after fully stirring for 2 hours, let the liquid stand for 50 minutes, use liquid separation to separate the lower aqueous phase, and collect a total of 110 g of the upper liquid, which is a dry pyrazoline compound;
[0085] 2) Under normal temperature conditions, transfer the dry pyrazoline compound obtained in step 1) to the reactor, stir at a stirring speed of 400rpm, and add 20g of potassium hydroxide to the reactor; liquid;
[0086] 3) Keep stirring the reaction solution continuously, heat the reaction solution to 180°C, keep it warm, and dist...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com