Preparation method of 4-aminocyclohexanol
A technology of aminocyclohexanol and aminophenol, which is applied in the field of preparation of 4-aminocyclohexanol, can solve the problems of long reaction time, low trans-isomer selectivity, polyacetate and the like, and achieves long catalyst life and catalytic effect. Excellent effect, high proportion of trans isomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
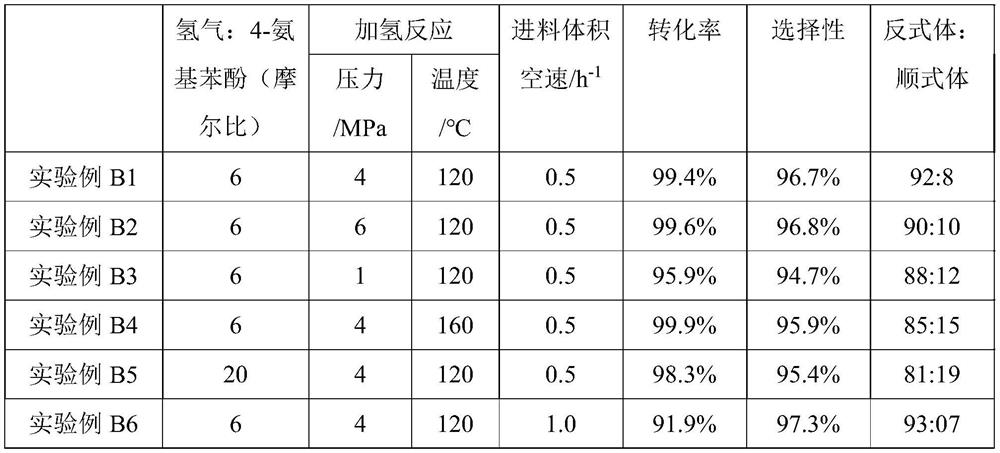
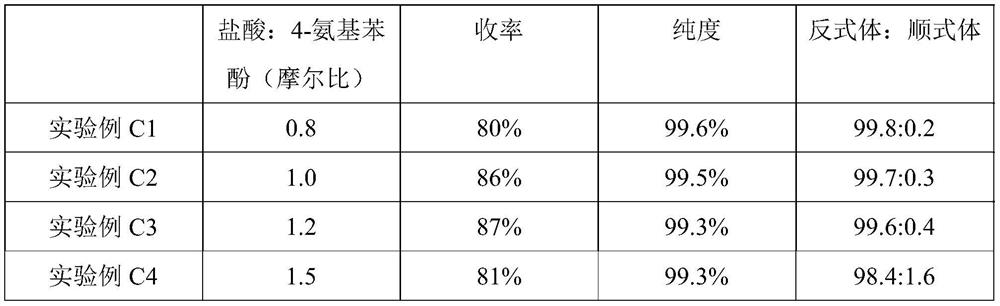
Examples
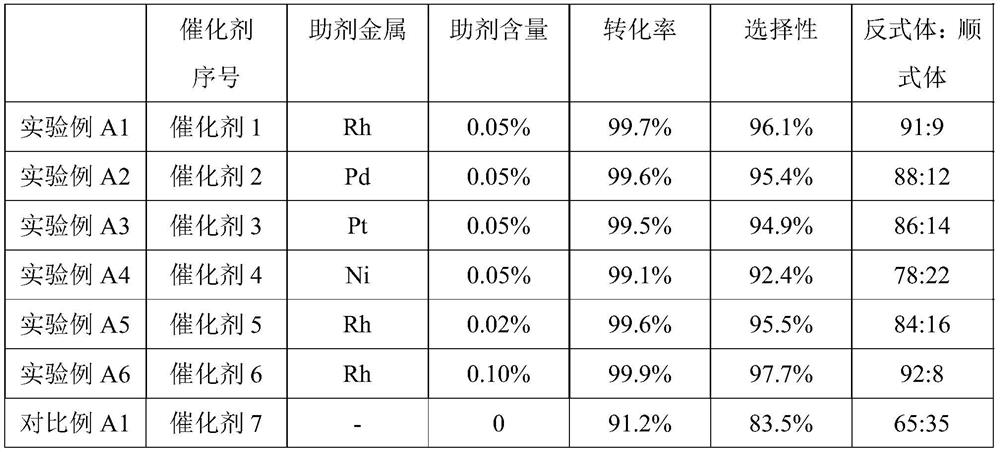
experiment example A1
[0018] Experimental example A1: Take a certain amount of ruthenium chloride and rhodium chloride, dissolve them with hydrochloric acid, and dissolve the corresponding amount of Al 2 o 3 Immerse in the above solution for 24 hours, bake at 120° C. for 8 hours, and bake at 400° C. in a muffle furnace for 4 hours. The loading of metal Ru is 0.5%, and the loading of metal Rh is 0.05%. Catalyst 1 is obtained.
experiment example A2
[0019] Experimental example A2: metal Ru and Pd were loaded, the loading amount of metal Ru was 0.5%, the loading amount of Pd was 0.05%, and the other was the same as that of Example Al to obtain catalyst 2.
experiment example A3
[0020] Experimental example A3: supporting metal Ru and Pt, the loading amount of metal Ru is 0.5%, the loading amount of Pt is 0.05%, and the other is the same as that of Example Al to obtain catalyst 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com