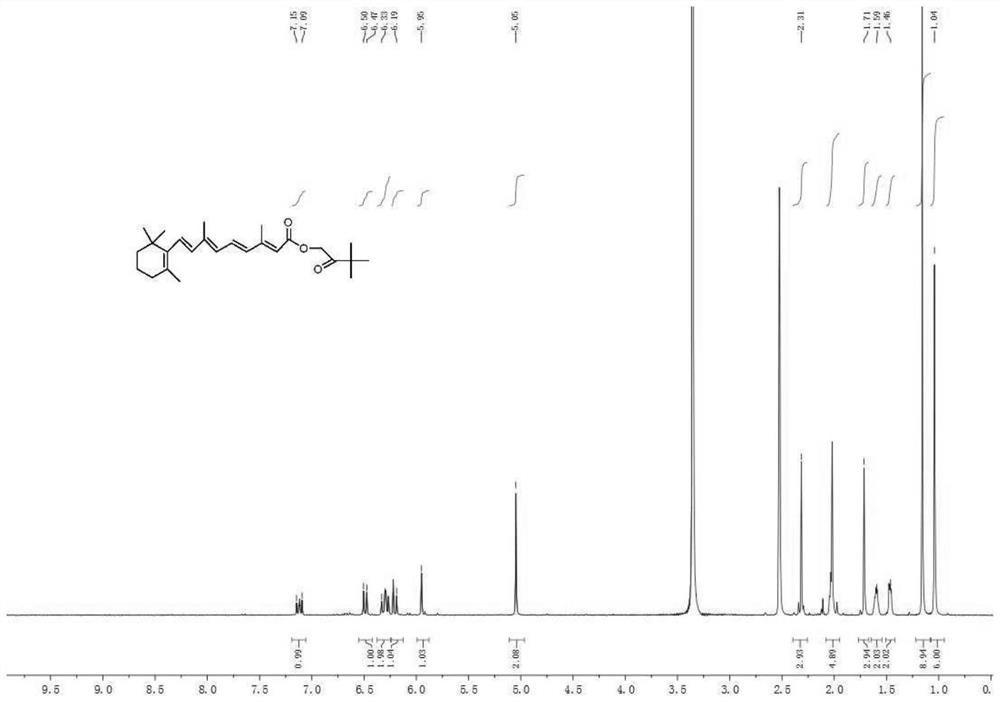
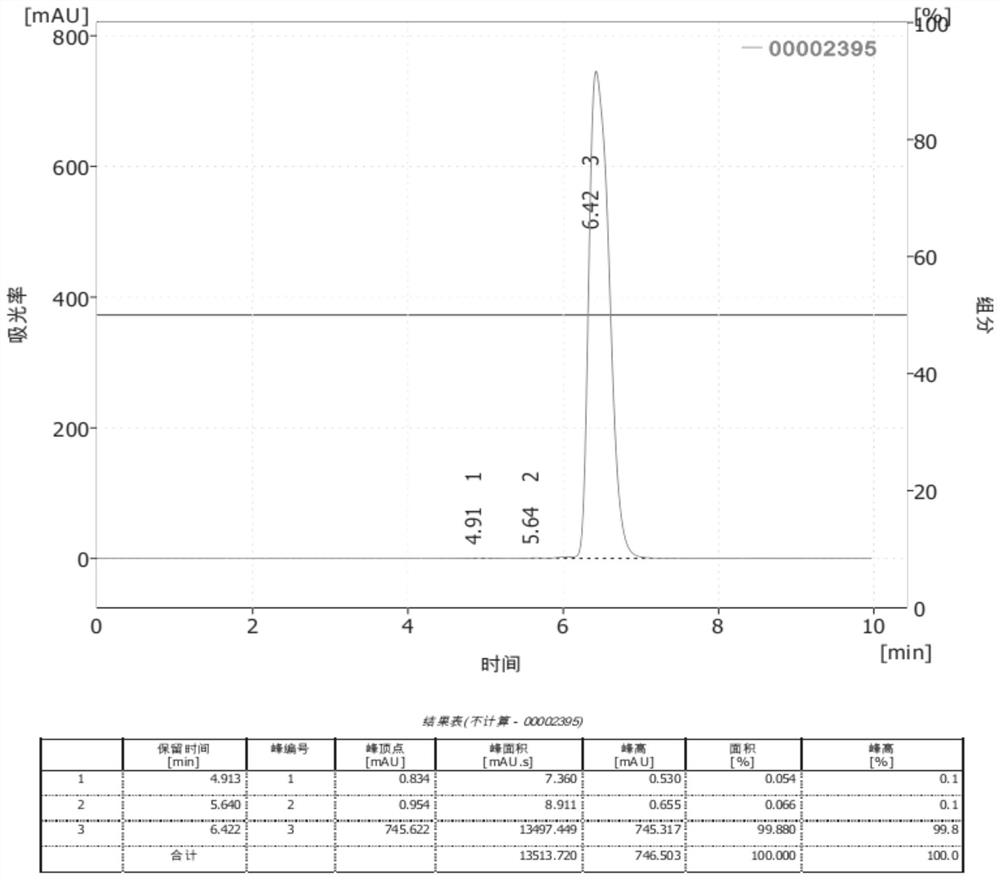
Synthesis method of hydroxyl pinacolone retinoate
A synthetic method, xanthate technology, applied in the direction of organic chemistry, etc., can solve the problems of high three waste treatment costs, product residues, low production efficiency, etc., to reduce the refining and purification of intermediates, low production costs, and high utilization of equipment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The synthesis method of hydroxy pinacol retinoic acid ester comprises the following steps:
[0034] S1: 20g(0.07mol) of retinoic acid, 100g(1.09mol) of toluene and 1g(0.014mol) of N,N- dimethylformamide are added into a 250ml three-necked flask in turn, and the temperature of the system is lowered to about 10℃ by ice bath after stirring;
[0035] S2, adding 5.8g(0.042mol) of phosphorus trichloride dropwise into the flask with a constant pressure dropping funnel under the protection of nitrogen, and after the dropping, raising the temperature of the system to 25℃ and keeping the temperature for 6h;
[0036] S3: after the liquid chromatograph detects that the reaction is complete, the lower inorganic solution is separated by standing, 10.5g(0.09mol) of 1- hydroxy -3,3- dimethylbutane -2- one is dripped in an ice bath, and after the dripping is finished, the temperature is raised to room temperature, 7.3g(0.07mol) of triethylamine is dripped slowly by a constant pressure dropp...
Embodiment 2
[0039] The synthesis method of hydroxy pinacol retinoic acid ester comprises the following steps:
[0040] S1: 20 g of retinoic acid (0.07 mol), 100 g of toluene (1.09 mol) and 2 g of N,N- dimethylformamide (0.028 mol) were added into a 250ml three-necked flask in turn, and the temperature of the system was lowered to about 10℃ by ice bath after stirring.
[0041] S2: adding 6.7g(0.049mol) of phosphorus trichloride dropwise into the flask with a constant pressure dropping funnel under the protection of nitrogen, and after the dropping, raising the temperature of the system to 30℃ and keeping the temperature for 6h;
[0042] S3: after the liquid chromatograph detects that the reaction is complete, the lower inorganic solution is separated by standing, 10.5g(0.09mol) of 1- hydroxy -3,3- dimethylbutane -2- one is dripped under the ice bath condition, after the dripping is finished, 8.5g(0.084mol) of triethylamine is slowly dripped at room temperature by a constant pressure dropping f...
Embodiment 3
[0050] The synthesis method of hydroxy pinacol retinoic acid ester comprises the following steps:
[0051] S1: 20 g of retinoic acid (0.07 mol), 100 g of toluene (1.09 mol) and 2 g of N,N- dimethylformamide (0.028 mol) were added into a 250ml three-necked flask in turn, and the temperature of the system was lowered to about 10℃ by ice bath after stirring.
[0052] S2: adding 6.7g(0.049mol) of phosphorus trichloride dropwise into the flask with a constant pressure dropping funnel under the protection of nitrogen, and after the dropping, raising the temperature of the system to 30℃ and keeping the temperature for 6h;
[0053] S3: after the liquid chromatograph detects that the reaction is complete, the lower inorganic solution is separated by standing, 10.5g(0.09mol) of 1- hydroxy -3,3- dimethylbutane -2- one is dripped under the ice bath condition, after the dripping is finished, 7.1g(0.07mol) of triethylamine is slowly dripped to room temperature by a constant pressure dropping fu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com