Euconafil oxide as well as preparation method and application thereof
A technique of Eucrena, quality control method, applied in the field of Eucrenafil oxide and its preparation, to achieve the effects of mild reaction conditions, easy availability of raw materials, and simple refining steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
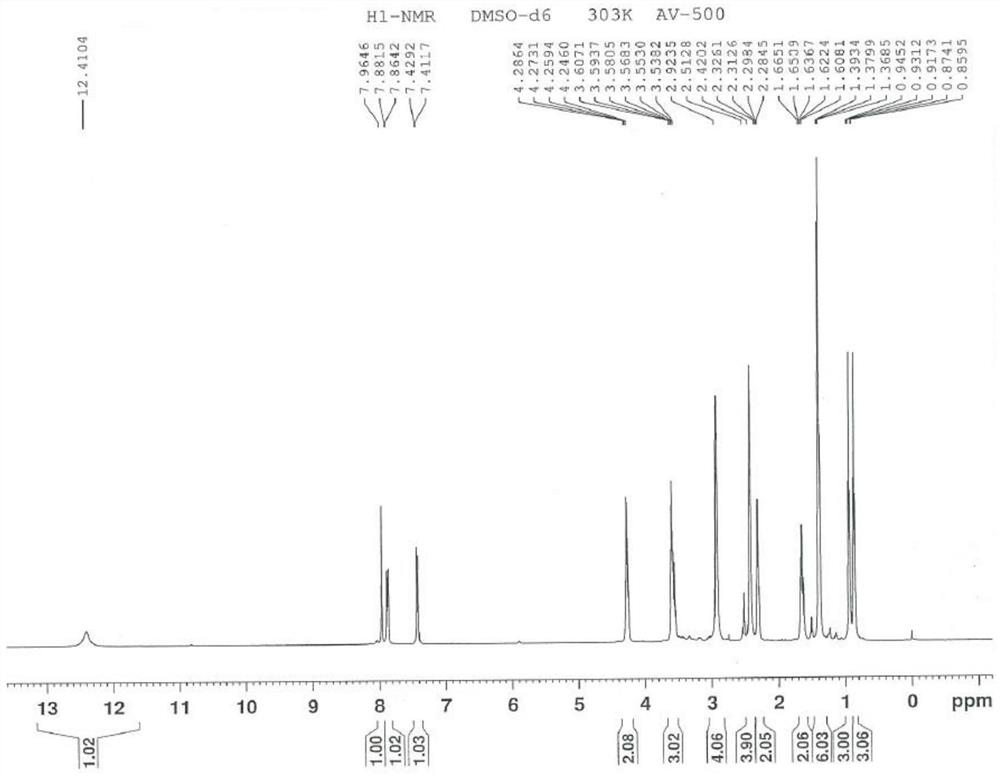
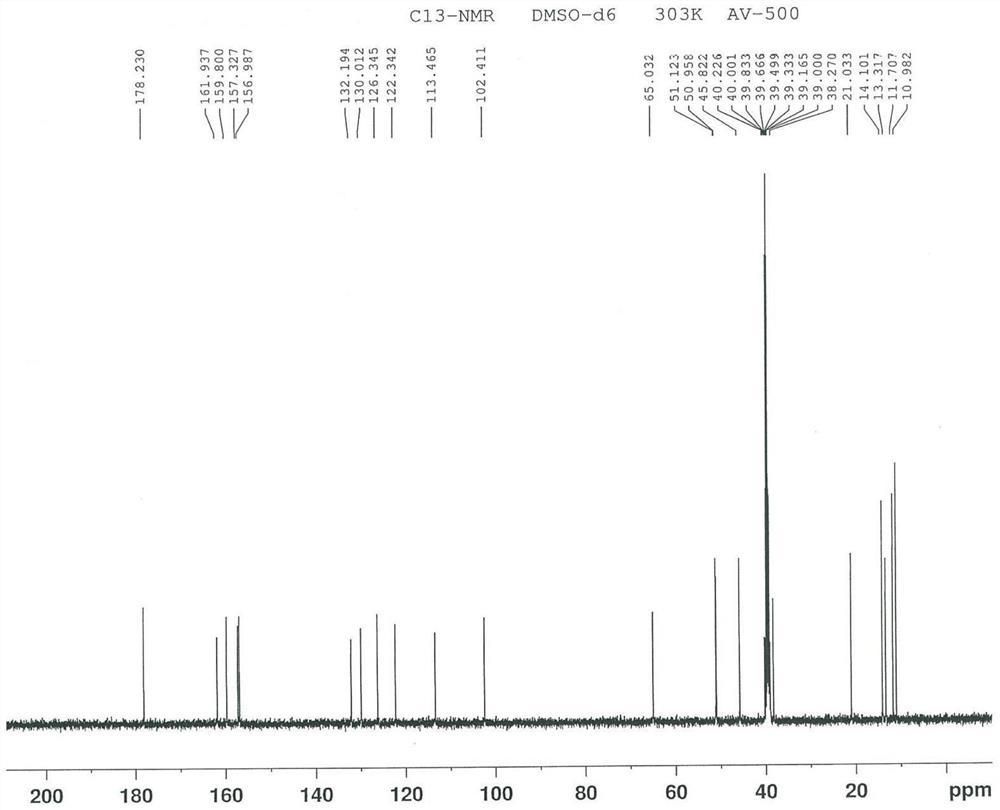
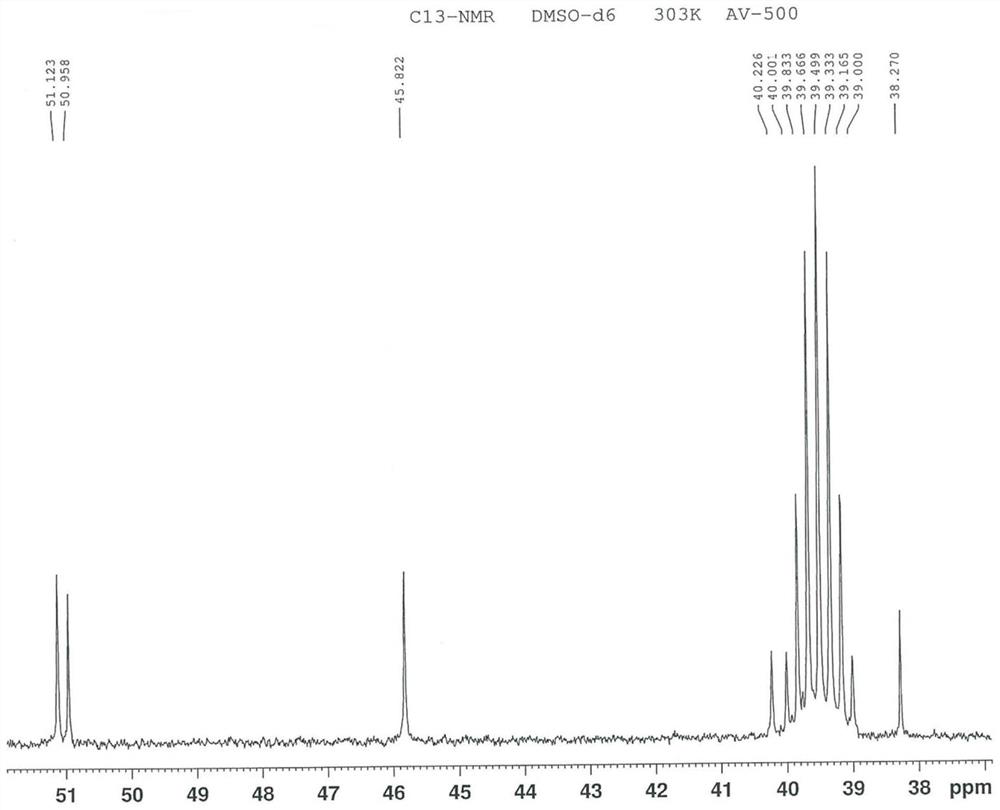
Image
Examples
Embodiment 1
[0095]
[0096] To 100 mL of formic acid, add 10 g of free base of ucnafil hydrochloride (that is, the compound shown in formula II, also known as ucnafil), stir to dissolve, cool down to -5 ~ 5 ℃, slowly add 4.5 g of 30% concentration After adding the hydrogen peroxide solution, the temperature was slowly raised to 70-80° C., the reaction was incubated for 5 h, and controlled by TLC (developing solvent: volume ratio of ethyl acetate and ethanol=5:1) until the reaction was completed. Add 100mL of 1mol / L sodium bisulfite solution to the reaction solution, stir for 30min, add 40mL of purified water and 200mL of dichloromethane, stir for 30min, and extract by liquid separation. After the obtained organic phase is washed with 100mL of saturated sodium chloride solution, Dry with anhydrous sodium sulfate for 2h. Filtration, the filtrate was concentrated to dryness under reduced pressure at 30~40°C, then 40 g of ethanol was added, the temperature was raised to 70~80°C, the system...
Embodiment 2
[0098]
[0099] To 45mL of formic acid, add 5g of the compound of the structure shown in formula II, stir and dissolve, cool to -5~5°C, slowly add 3.0g hydrogen peroxide solution with a concentration of 30%, be warming up to 70~80°C after adding, and keep the reaction 4h, controlled by TLC (developing solvent: volume ratio of ethyl acetate and ethanol=5:1) to completion of the reaction. 50mL of 1mol / L sodium bisulfite solution was added to the reaction solution, stirred for 30min, added with 25mL of purified water and 100mL of dichloromethane, stirred for 30min, and subjected to liquid separation extraction. The obtained organic phase was washed with 50mL of saturated sodium chloride solution, and then Dry with anhydrous sodium sulfate for 2h. Filtration, the filtrate was concentrated to dryness under reduced pressure at 30-40 °C, 25 g of ethanol was added, the temperature was raised to 70-80 °C, the system was dissolved and then cooled to -5-5 °C, crystallized, filtered, a...
Embodiment 3
[0101]
[0102] In 45mL of acetic acid, add 5g of the compound of the structure shown in formula II, stir and dissolve, cool down to -5~5°C, slowly add 3.0g hydrogen peroxide solution with a concentration of 30%, be warming up to 70~80°C after adding, and keep the reaction 5h, controlled by TLC (developing solvent: volume ratio of ethyl acetate and ethanol=5:1) to completion of the reaction. 50mL of 1mol / L sodium bisulfite solution was added to the reaction solution, stirred for 30min, added with 25mL of purified water and 100mL of dichloromethane, stirred for 30min, and subjected to liquid separation extraction. The obtained organic phase was washed with 50mL of saturated sodium chloride solution, and then Dry with anhydrous sodium sulfate for 2h. Filtration, the filtrate was concentrated to dryness under reduced pressure at 30-40 °C, 25 g of ethanol was added, the temperature was raised to 70-80 °C, the system was dissolved and then cooled to -5-5 °C, crystallized, filter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com