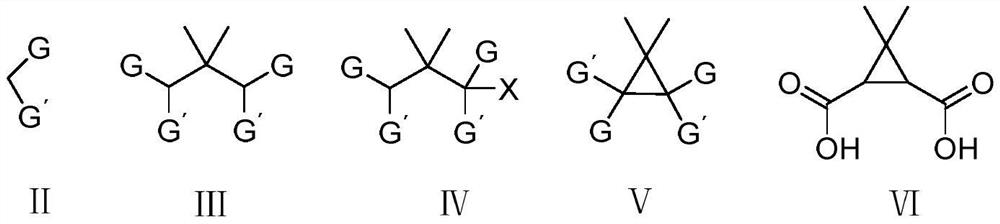
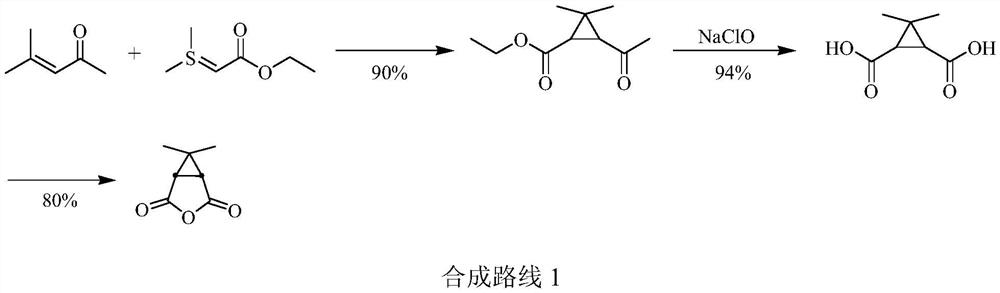
Low-cost preparation method of calonic acid and calonic anhydride
A caronic acid and acid hydrolysis technology, applied in the preparation of carboxylate/lactone, organic chemistry, etc., can solve the problems of low product yield, poor operation safety, and high raw material price, and achieve specific reaction selectivity and reduce Online concentration, selective and specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Embodiment 1: the preparation of carronic acid (Ⅵ)
[0087] To a 1000 ml four-neck flask connected with stirring, thermometer, water separator and reflux condenser, add 500 g of toluene, 46.0 g (0.8 mole) of acetone, 99.1 g (1.0 mole) of methyl cyanoacetate (II 1 ), 0.6 gram of piperidine, 0.4 gram of acetic acid, under stirring condition, heat 82-85 ℃ to reflux and separate water for 3 hours; Stir and react at -45°C for 3 hours; after cooling down to room temperature, filter, and transfer the obtained filtrate to a constant pressure dropping funnel for use. In another 2000 ml four-necked flask connected with a stirring and thermometer, add 120 g (0.6 moles) of 27% sodium methoxide methanol solution, keep the inner temperature between 30-35 ° C, and add dropwise to a constant pressure dropping funnel under stirring The filtrate in about 3 hours was added dropwise. Thereafter the reaction was stirred at 35-40°C for 2 hours. Add 300 grams of water, 200 grams of 40% aqu...
Embodiment 2
[0091] Embodiment 2: the preparation of karonic acid (Ⅵ)
[0092] Add 400 g of n-hexane, 30.0 g (0.5 mole) of acetone, 66.0 g (1.0 mole) of malononitrile (II 2 ), 0.6 gram of piperidine, 0.8 gram of benzoic acid, under the condition of stirring, heat 60-65 ℃ to reflux and divide water for 4 hours; Stir and react at 40-45°C for 3 hours; after cooling down to room temperature, filter, and transfer the resulting filtrate to a constant pressure dropping funnel for use. In another 2000 ml four-necked flask connected with a stirring and thermometer, add 120 g (0.6 moles) of 27% sodium methoxide methanol solution, keep the inner temperature between 30-35 ° C, and add dropwise to a constant pressure dropping funnel under stirring The filtrate in about 3 hours was added dropwise. Thereafter the reaction was stirred at 35-40°C for 2 hours. Add 300 grams of water, 200 grams of 40% aqueous sodium hydroxide solution, stir and react at 80-85° C. for 3 hours, and distill out n-hexane and me...
Embodiment 3
[0093] Embodiment 3: the preparation of carronic acid (Ⅵ)
[0094] Add 600 g of ethanol, 30.0 g (0.5 mole) of acetone, 113.0 g (1.0 mole) of ethyl cyanoacetate (II 3 ), 0.6 gram of piperidine, 0.4 gram of acetic acid, under the condition of stirring, heat 75-80 ℃ of reflux with water for 3 hours; Stir and react at -35°C for 3 hours; after cooling down to room temperature, transfer the resulting filtrate to a constant pressure dropping funnel for use. In another 2000 ml four-necked flask connected with a stirring and thermometer, add 200 g of ethanol and 150 g (1.1 moles) of potassium carbonate, keep the internal temperature between 50-55 ° C, and drop them into the constant pressure dropping funnel under stirring The filtrate was added dropwise in about 3 hours. Thereafter the reaction was stirred at 50-55°C for 2 hours. Add 300 grams of water, 200 grams of 40% aqueous sodium hydroxide solution, stir and react at 80-85° C. for 3 hours, and simultaneously distill off ethanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com