Simple preparation method of 2,3-dichloropyridine
A technology of dichloropyridine and chlorobenzene is applied in the field of simple preparation of 2,3-dichloropyridine, can solve the problems of high price of 2-chloronicotinamide, unsuitable for industrialization and the like, and achieves short process route, easy operation and reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
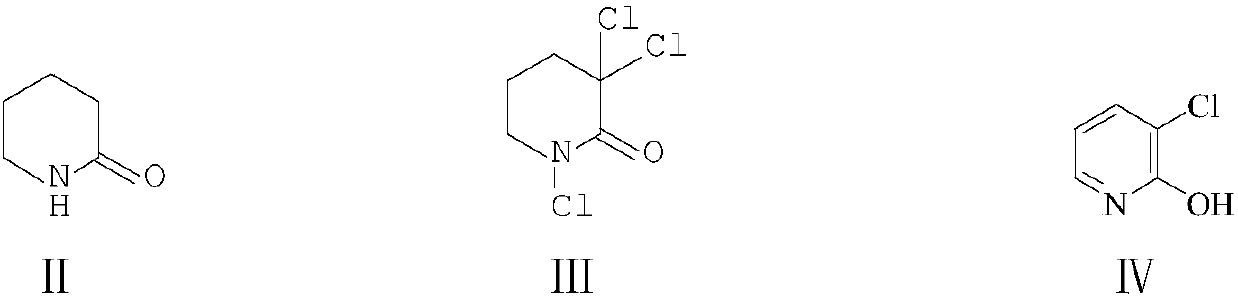
[0046] Embodiment 1: the preparation of 3-chloro-2-hydroxypyridine (IV)
[0047] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, reflux condenser, gas guide tube and 30wt% sodium hydroxide aqueous solution absorbing device, add 160 grams of 1,2-dichloroethane, 49.5 grams (0.5 moles) of 2- Piperidone (II), 0.6 g of triethyl phosphite, heat, keep between 50-60 °C, slowly pass in 120.0 g (1.7 moles) of chlorine gas, and pass in about 3-4 hours, then 55-60 Stir and react at ℃ for 4 hours, cool to 20-25°C, add 160.0 grams (1.6 moles) of 40wt% sodium hydroxide aqueous solution, stir and react at 50-55°C for 3 hours, cool to 20-25°C, acidify the pH of the system with 30wt% hydrochloric acid 3.0-4.0, separate layers, extract the water layer with 1,2-dichloroethane 3 times, 50 grams each time, combine the organic phases, recover the solvent by distillation, and dry to obtain 59.7 grams of 3-chloro-2-hydroxypyridine as a solid (Ⅳ), the yield is 92....
Embodiment 2
[0048] Embodiment 2: the preparation of 3-chloro-2-hydroxypyridine (IV)
[0049] In the 500 milliliter four-neck flask that is connected with stirring, thermometer, reflux condenser, constant pressure dropping funnel and 30wt% sodium hydroxide aqueous solution absorption device, add 50 grams of dichloromethane, 9.9 grams (0.1 mole) 2-piperidine Ketone (II), 0.2 grams of 4-dimethylaminopyridine, 45.0 grams (0.43 moles) of 35wt% hydrochloric acid, kept at 35-40 °C and added dropwise 45.0 (0.4 moles) of 30wt% hydrogen peroxide, and the addition was completed in about 3-4 hours , then stirred at 40-45°C for 5 hours, cooled to 20-25°C, added 35.0 grams (0.35 moles) of 40wt% sodium hydroxide aqueous solution, stirred and reacted at 40-45°C for 3 hours, cooled to 20-25°C, 30wt% The pH value of the hydrochloric acid acidification system was 3.0-4.0, and the layers were separated, and the aqueous layer was extracted with dichloromethane 3 times, 50 g each time, the organic phases were ...
Embodiment 3
[0050] Embodiment 3: the preparation of 3-chloro-2-hydroxypyridine (IV)
[0051] In the 500 milliliters of four-neck flasks that are connected with stirring, thermometer, reflux condenser, gas guide tube and 30wt% sodium hydroxide aqueous solution absorption device, add 150 gram trichloroethanes, 49.5 grams (0.5 moles) 2-piperidone ( Ⅱ), 0.9 grams of triphenyl phosphite, heated, kept between 70-75 °C, slowly introduced 115.0 grams (1.62 moles) of chlorine gas, and the introduction was completed in about 3-4 hours, then stirred at 70-75 °C for 3 hour, cooled to 20-25°C, added 170.0 grams (1.7 moles) of 40wt% sodium hydroxide aqueous solution, stirred and reacted at 40-45°C for 3 hours, cooled to 20-25°C, and the pH value of the 30wt% hydrochloric acid acidification system was 3.0-4.0 , layered, the aqueous layer was extracted 3 times with trichloroethane, each 50 grams, the organic phases were combined, the solvent was reclaimed by distillation, and dried to obtain 60.4 grams o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com