Application of combined medication of bacteroides fragilis zwitterionic capsular polysaccharide and immune checkpoint inhibitor to treatment of genitourinary system tumors
A technology of Bacteroides fragilis and immune checkpoint, which is applied in the field of biomedicine to prevent and treat genitourinary system tumors and enhance the body's anti-tumor immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
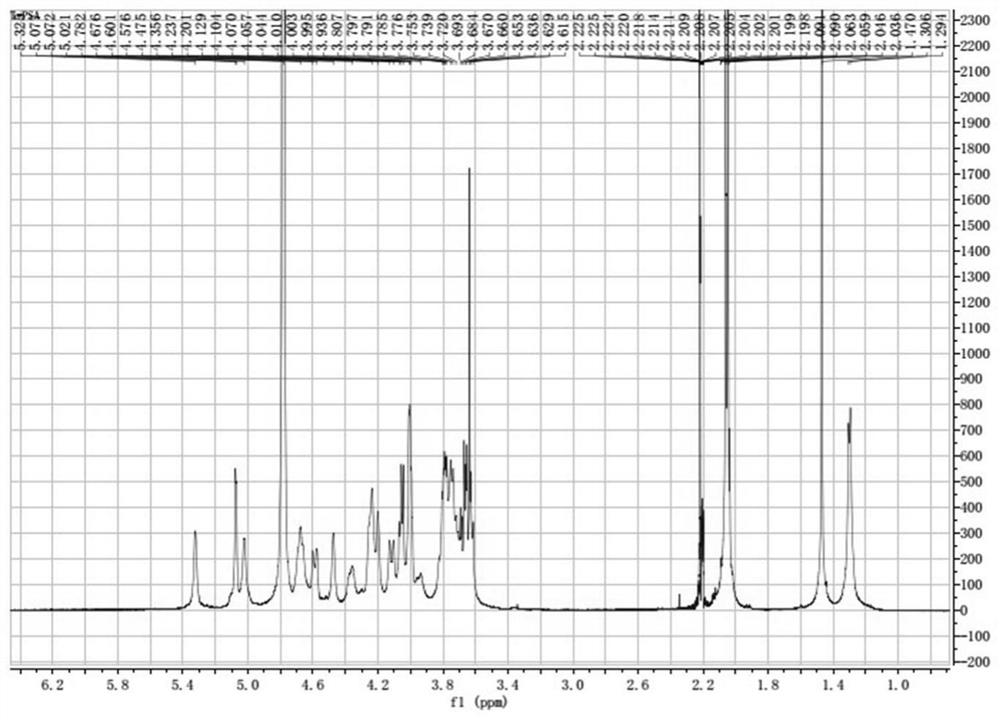
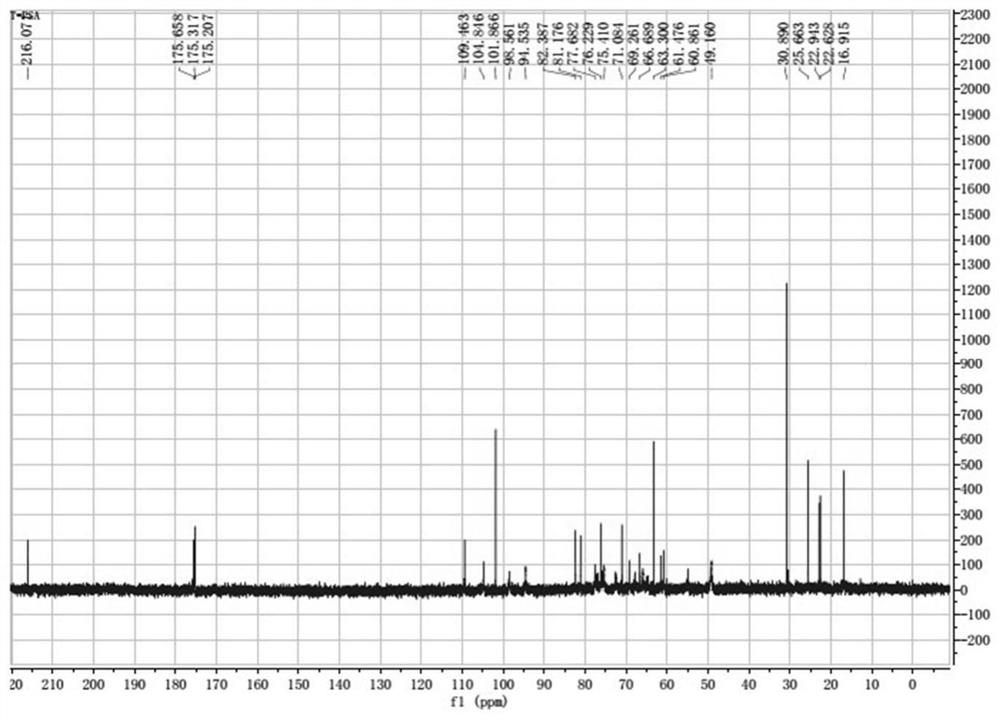
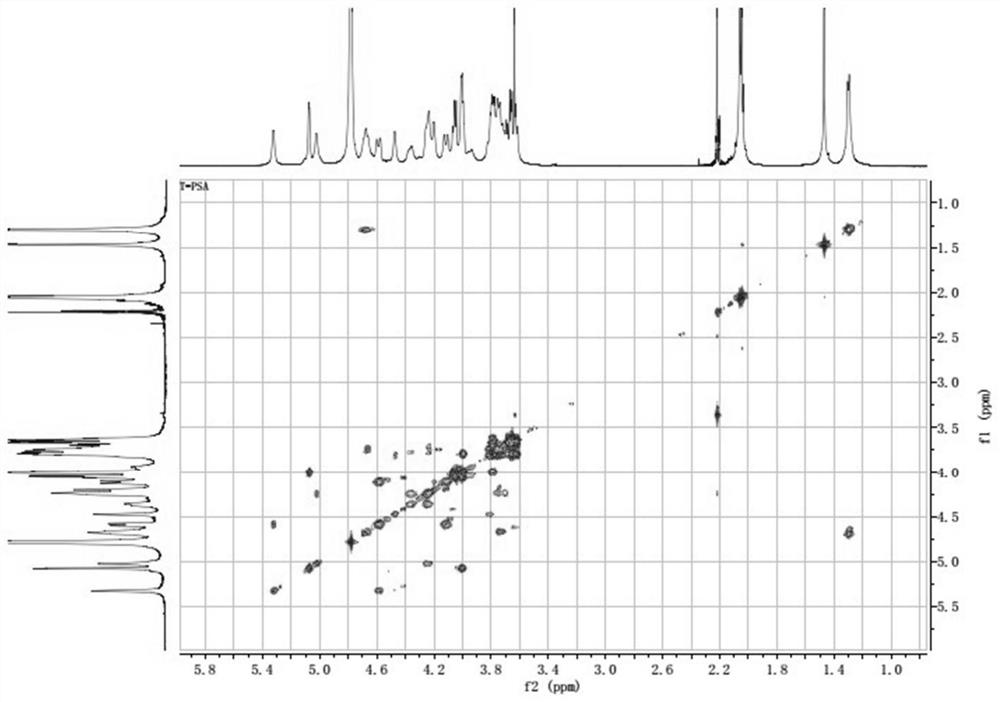
[0093] Example 1 Preparation of Bacteroides fragilis capsular polysaccharide A
[0094] (1) Streak-inoculate Bacteroides fragilis ZY-312 strains on blood plates, culture anaerobically for 48 hours, select a single colony and inoculate it in plant-derived peptone liquid medium for fermentation and culture for 8 hours (at a temperature of 37°C), and the obtained bacteria The solution was centrifuged and precipitated at 3000r / min, centrifuged for 15min, the supernatant was removed, and the precipitate was collected to obtain the Bacteroides fragilis ZY-312 sludge.
[0095] (2) Take 50g of the bacteria slime prepared in step (1), add 300g of purified water to resuspend the bacteria, adjust the pH to 3.5 with 1mol / L hydrochloric acid solution, extract at 100°C for 1.5h, cool to room temperature, and centrifuge at 12000g for 10min at room temperature , take the supernatant to obtain the crude sugar solution;
[0096] (3) The crude sugar solution is concentrated by ultrafiltration t...
Embodiment 2
[0099] Example 2 Drug efficacy test of Bacteroides fragilis zwitterionic capsular polysaccharides and immune checkpoint inhibitors in synergistic treatment of Renca renal carcinoma xenografts in mice
[0100] 1. Experimental design and process
[0101] (1) Animal modeling
[0102] Take 100 Kunming mice, male, SPF grade, body weight (20±2) g, and 80 of them were subcutaneously inoculated with 250 μL of Renca cells (concentration of 1×10 7 The tumor growth of the mice was observed every day, and tumor nodules of about 5 mm were seen at the injection site after 7 days, which was considered as a successful establishment of the renal cancer tumor-bearing mouse model.
[0103] (2) Animal grouping and administration
[0104] 60 mice successfully modeled were taken and randomly divided into 6 groups, 10 in each group, model group, capsular polysaccharide A (PSA, 100 μg / mouse, prepared by Example 1, the same below), PD-1 antibody (PD-1ab, product number BE0146, purchased from BioXcell...
Embodiment 3
[0117] Example 3 Application of Bacteroides fragilis Zwitterionic Capsular Polysaccharide and Immune Checkpoint Inhibitors in Synergistic Treatment of Transplanted Tumors of Bladder Cancer (Urothelial Carcinoma) in Mice
[0118] 1. Experimental design and process
[0119] (1) Animal modeling
[0120] Take 100 male BALB / C-nude mice, among which 80 nude mice were subcutaneously disinfected on the back, and each was inoculated with 0.2ml T24 cell suspension (the cell concentration was 2×10 7 / mL). After transplantation, the nude mice were kept in the SPF environment, and tumor nodules appeared at the inoculation site, and the texture was hard and other indicators were identified as tumor formation.
[0121] (2) Animal grouping and administration
[0122] 60 nude mice successfully modeled were randomly divided into 6 groups, 10 in each group, model group, capsular polysaccharide A (PSA, 100 μg / mouse) group, PD-1 antibody group (PD-1ab, BE0146, BioXcell 200 μg / mouse) and low (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com